Abstract
Purpose
The prognostic significance of perineural invasion by prostate cancer is debated. We investigated the association between perineural invasion and clinicopathological factors and the effect of perineural invasion on survival in patients with prostate cancer.
Materials and Methods
A total of 361 patients with prostate cancer without any neoadjuvant therapies prior to surgery from 1999 to 2010 were analyzed retrospectively. Whole-mount sections of surgical specimens from all patients who underwent radical prostatectomy were evaluated. Positive perineural invasion was defined as infiltration of cancer cells in the perineurium or neural fascicles. The relationship of perineural invasion with clinicopathological features and prognosis of prostate cancer was studied. We also researched preoperative factors that were associated with perineural invasion.
Results
Perineural invasion in a prostatectomy specimen (PNIp) was positive in 188 of 361 patients (52.1%). In the multivariate analysis of the preoperative variables, PNIp was related to the primary Gleason grade (p=0.020), the number of positive cores (p=0.008), and the percentage of tumor cells in positive cores (p=0.021), but not to perineural invasion of a prostate biopsy. In the evaluation between PNIp and pathologic findings of the prostatectomy specimen, PNIp was related to the Gleason score (p=0.010), T-stage (p=0.015), and lymphovascular invasion (p=0.019). However, by multivariate analysis, the PNIp was not an independent prognostic factor of biochemical serum recurrence (p=0.364) or cancer-specific survival (p=0.726).
A distinctive feature of cancer cells is their ability to dissociate from the primary tumor and establish metastases at various sites. The common routes by which cancer cells spread include local invasion, and lymphatic, hematogenous, and peritoneal dissemination. The hematogenous and lymphatic pathways are well-established routes of metastatic spread. However, although routes along nerves have been described in the literature since the mid-1800s, they have received relatively little research attention [1]. Recently, it has become increasingly evident that cancer-stromal interactions play a critical role in cancer growth and progression. The involvement of peripheral nerves has been overlooked for a long time; these nerves have been considered inert bystanders in solid malignancies, but they are now gaining recognition as potentially important components of the cancer microenvironment. Cancer cells invade both the epineurium and the perineurium, and may reach the endoneurium, becoming intimately associated with Schwann cells and nerve axons [1]. Perineural invasion was first reported in the English literature by doctors who described head and neck cancers with a predilection for growth along nerves, as they made their way toward the intracranial fossa [2].
The clinical significance of perineural invasion is controversial. D'Amico et al showed that perineural invasion is an independent prognostic factor for prostate cancer recurrence [3]. However, Freedland et al reported that perineural invasion did not correlate with extracapsular extension or biochemical recurrence [4].
We compared the preoperative prediction of perineural invasion in a prostate biopsy (PNIb) with that of perineural invasion in a prostatectomy specimen (PNIp) and investigated the clinical and prognostic significance of PNIp in prostate cancer patients.
We reviewed the medical records of 361 consecutive patients with prostate adenocarcinomas who had received no hormonal therapy or radiation therapy before or after a retropubic radical prostatectomy or laparoscopic radical prostatectomy, performed between 1999 and 2010. Data pertaining to the demographics, staging, pathology, and outcomes of each patient were reviewed and entered into a comprehensive database. All procedures involving study participants were approved by the Institutional Review Board (IRB approved protocol number: E2010-014). The period of observation of this unselected cohort was the interval between the date of the surgical resection and the last contact (death or last follow-up). The mean duration of follow-up was 42.4±33.6 months (range, 6.5-141.6 months).
The patient characteristics and pathological parameters are listed in Table 1. The mean patient age at the time of radical prostatectomy was 69.0 years (range, 49-94 years). Prostate biopsies were recommended for men with a serum prostate-specific antigen (PSA) level of 3.0 ng/ml or higher. We evaluated the primary Gleason grade, secondary Gleason grade, Gleason score, occurrence of a high Gleason grade (>3), percentage of positive biopsy cores, percentage of tumor cells in the positive cores, bilaterality, and perineural invasion in a prostate biopsy. The percentage of positive biopsy cores was defined as: (number of positive biopsy cores/total number of biopsy cores) ×100. We analyzed the association between the preoperative parameters cited above and PNIp to evaluate the preoperative prediction of PNIp.
Either a medical or a surgical oncologist who was a member of our institution's multidisciplinary tumor board at the time of the patient's treatment established the tumor stage postoperatively, according to the tumor, lymph node, metastasis (TNM) classification of the International Union Against Cancer (UICC 2002) and the Gleason system [5]. We reviewed the primary Gleason grade, secondary Gleason grade, Gleason score, presence of a high Gleason grade (>3), presence of Gleason grade 5, T-stage, tumor volume, lymphovascular invasion, surgical margin status, perineural invasion, pelvic lymph-node metastasis at radical prostatectomy, and nadir PSA. Tumor volume was defined as the volume of cancer cells relative to the total resected prostate volume. Perineural invasion was defined as tumor cells within any layer of the nerve sheath or tumor cells in the perineural space that involved at least one third of the nerve circumference. Lymphovascular invasion was defined as the presence of cancer cells within an endothelium-lined space without a muscular wall.
Nadir serum PSA was checked 4-6 weeks after the radical prostatectomy. We also followed up the serum PSA every 3 months during the first year after surgery, semiannually in years 2 to 5, and annually thereafter. The cutoff value for biochemical serum PSA recurrence was defined as 0.2 ng/ml.
Survival records were obtained from the Korean National Statistics Registry Database, and the cancer-specific survival data were analyzed.
All radical prostatectomy specimens were evaluated in a standard manner. After eliminating the apical and bladder neck margins, the specimens were sectioned transversely at 5 mm intervals from the apex to the base. The seminal vesicles were evaluated at the intersection at which they entered the prostate gland. Whole-mount sections (5 µm thick) were stained with H&E. The apical and bladder neck margins and the anterior, radial, posterior, and posterolateral surgical margins were defined as positive if the tumor was in direct contact with the indicated inked surface of the prostate in the sections.
The original H&E stained slides from the tumor resections were collected from the pathology department. All slides containing tumor cells were reviewed for PNIp by a single pathologist with expertise in PNIp, who was blinded to all patient data, including the stage of the disease and its outcome.
The associations between the preoperative parameters and PNIp were calculated with a binary logistic regression model and the Mantel-Haenszel χ2 test. The association of PNIp with various clinicopathological characteristics was assessed by using the (two-tailed) Pearson correlation test. The influence of PNIp on biochemical recurrence and overall and cancer-specific survival were estimated by using the Kaplan-Meier method with a log-rank test. The effects of PNIp and other clinicopathological parameters on biochemical recurrence and cancer-specific survival were analyzed by using the Cox regression model. All analyses were performed with SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). All results were considered significant at p<0.05.
Fifty-two percent of patients (188 of 361 patients) had a positive PNIp (Fig. 1). Only 7.4% of these patients (14 of 188 patients) were identified as having positive PNIb, demonstrating the underreporting of this pathology in prostate biopsies.
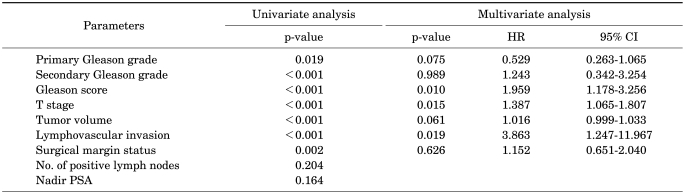
In evaluating the relations between the preoperative variables and PNIp, PNIp was related to preoperative PSA (p=0.002), the primary Gleason grade (p<0.001), secondary Gleason grade (p=0.003), Gleason score (p<0.001), a Gleason grade >3 (p<0.001), the number of positive cores (p<0.001), the percentage of positive cores (p<0.001), and the percentage of tumor cells in positive-core prostate biopsies (p<0.001), but not to age (p=0.069), bilaterality (p=0.175), or perineural invasion at prostate biopsy (p=0.277). In a multivariate analysis, PNIp was related to the primary Gleason grade (p=0.020, hazard ratio=2.040, 95% confidence interval [CI] 1.119-3.719), the number of positive cores (p=0.008, hazard ratio=1.303, 95% CI: 1.072-1.582), and the percentage of tumor cells in positive cores (p=0.021, hazard ratio=1.015, 95% CI: 1.002-1.029) at prostate biopsy.
In a univariate analysis, PNIp was correlated with the pathological stage of the prostatectomy specimen (p<0.001). Perineural invasion was observed in 50% of T2a tumors, 11.1% of T2b tumors, 48.3% of T2c tumors, 79.4% of T3a tumors, 91.9% of T3b tumors, and 100% of T4 tumors. The Gleason score and the primary and secondary Gleason grades were also significantly related to PNIp (p<0.001, p=0.019, and p<0.001, respectively). PNIp was observed in 39.3% of patients with a Gleason grade of ≤3 and in 71.5% of patients with a Gleason grade of >3. PNIp was observed in 81.3% of patients with a Gleason grade of 5.
The tumor volume of the prostate cancer was higher in the PNIp-positive specimens than in the PNIp-negative specimens (27.7% vs 14.5%, respectively; p<0.001). PNIp was observed almost twice as frequently in surgical-margin-positive specimens at the time of radical prostatectomy than in the surgical-margin-negative specimens (53.5% vs 35.7%, respectively; odds ratio 2.069, p=0.003). PNIp showed a slight correlation with lymph-node metastasis (5.3% vs 0.8%, respectively; odds ratio: 7.022, p=0.055). In a multivariate analysis, PNIp was significantly related to the Gleason score (p=0.010, hazard ratio=1.959, 95% CI: 1.178-3.256), T-stage (p=0.015, hazard ratio=1.387, 95% CI: 1.065-1.807), and lymphovascular invasion (p=0.019, hazard ratio=3.863, 95% CI: 1.247-11.967).
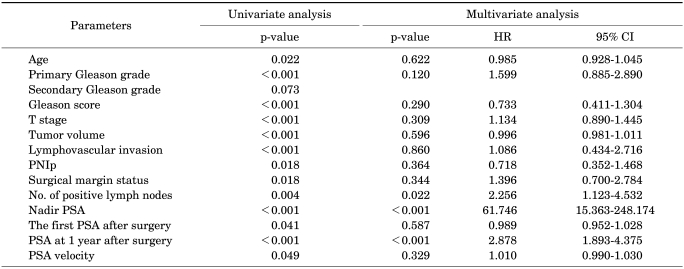
The relations between PNIp and other clinical and pathological parameters were investigated with the Pearson correlation. Age (p=0.022), primary Gleason grade (p<0.001), Gleason score (p<0.001), tumor stage (p<0.001), tumor volume of the prostate (p<0.001), lymphovascular invasion (p<0.001), PNIp (p=0.018), surgical margin status (p=0.018), number of positive lymph nodes (p=0.004), nadir PSA (p<0.001), the first PSA after surgery (p=0.041), PSA at 1 year after surgery (p<0.001), and PSA velocity (p=0.049) were significantly related to biochemical serum PSA recurrence. Using the log-rank test, we found no significant difference in biochemical serum PSA recurrence between patients with PNIp and those without PNIp (p=0.597; Fig. 2). In a multivariate analysis, nadir PSA (p<0.001, hazard ratio=61.746, 95% CI: 15.363-248.174) and PSA at 1 year after surgery (p<0.001, hazard ratio=2.878, 95% CI: 1.893-4.375) were significantly related to biochemical serum PSA recurrence. However, PNIp was not correlated with biochemical recurrence (p=0.364).
In a Kaplan-Meier survival analysis, the Gleason score, preoperative PSA, and nadir PSA were significantly related to cancer-specific survival (p<0.001, p<0.001, and p<0.001, respectively). However, PNIp did not correlate with cancer-specific survival (p=0.726; Fig. 3).
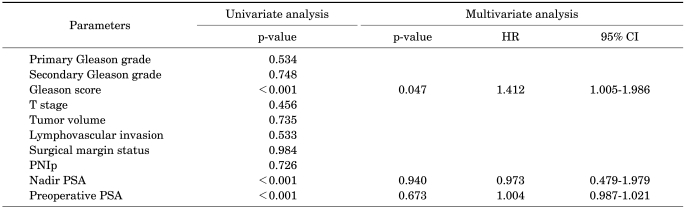
A Cox proportional multivariate analysis was used to assess the influence of all the significant parameters on cancer-specific survival and 5-year PSA-recurrence-free survival. The Gleason score was significantly associated with a poor prognosis (p=0.047, hazard ratio=1.412, 95% CI: 1.005-1.986). The presence of PNIp did not correlate with biochemical recurrence or cancer-specific survival.
Perineural invasion has become an increasingly relevant, yet understudied, aspect of tumor biology in several cancers, including prostate cancer. The role of perineural invasion and its utility to clinicians continue to be debated [6]. Because the incidence of prostate cancer is increasing rapidly in Korea [7], we sought to determine the impact of perineural invasion on prostate cancer in our patient population.
Previous studies have reported incidences of PNIb of 7-43% and of PNIp of 31.9-79.0% [3,8-12]. In the present study, PNIb and PNIp were observed in 7.4% and 52.8% of patients, respectively, which means that PNIb has only limited prognostic value and more attention should be directed to pathological examinations. However, PNIp was related to the primary Gleason grade, secondary Gleason grade, Gleason score, Gleason grade of >3, the number of positive cores, the percentage of positive cores, and the percentage of tumor cells in the positive cores on prostate biopsies. This result indicates that biopsy specimens do not represent the whole prostate pathology, even when multiple prostate biopsies are performed.
The clinical significance of PNIb is controversial. Some reports have shown that PNIb does not predict biochemical PSA recurrence after radical prostatectomy [4,9]. However, other reports have demonstrated that PNIb is significantly related to postoperative biochemical recurrence [8,13]. Loeb et al showed that PNIb is an independent risk factor for aggressive pathological features and a nonindependent risk factor for biochemical progression after radical prostatectomy [8]. A limitation of our study was that we could not assess the exact predictive value of PNIb for biochemical recurrence because the number of patients with positive PNIb was small.
Lee et al showed a statistically significant association between the presence of PNIp and adverse preoperative risk parameters, including a higher clinical T-stage, higher Gleason score at biopsy, and higher preoperative serum PSA [14]. Another report also demonstrated that PNIp was significantly related to preoperative serum PSA and PSA density [15]. In our study, we found that PNIp was significantly related to the primary Gleason grade, the percentage of positive cores, and the percentage of positive cores on prostate biopsy.
Recently, Jeon and colleagues also reported that PNIp was related to a higher Gleason score, extracapsular extension, seminal vesicle invasion, and a positive surgical margin [15]. Stone et al reported that PNIp predicted pelvic lymph node metastasis in men with prostate cancer [16]. In the present study, we also found that PNIp correlated with several clinicopathological parameters, including the pathological T-stage, Gleason score, and lymphovascular invasion.
Stone et al showed that PNIp is an independent predictor of lymph node metastasis in prostate cancer patients [16]. Another report suggested PNIp as a marker for pathologically advanced prostate cancer [14]. However, Miyake et al reported that PNIp is not an independent predictor of biochemical recurrence and might not provide any extra useful information when the presence of perineural invasion is considered in predicting the prognosis of men undergoing radical prostatectomy if there are other conventional parameters available [17]. Merrilees et al also suggested that PNIp does not predict biochemical serum PSA recurrence [18]. Our data indicate that PNIp did not correlate with biochemical serum PSA recurrence, although it was related to other known prognostic factors.
There are a few reports of the significance of perineural invasion for the survival of patients with prostate cancer [1]. However, most of these reports have limitations, because the studies included patients who had undergone radiation therapy, so they evaluated the presence of PNIb, but not PNIp. Therefore, we could not compare our results with previous reports, including biochemical serum PSA recurrence or the survival rate, in patients who had undergone radical prostatectomy. Moreover, most studies have defined biochemical recurrence as the only study endpoint, whereas we evaluated the prognostic value of PNIp for cancer-specific survival. In our study, PNIp was not significantly related to cancer-specific survival. In this study, the relationship between PNIp and biochemical recurrence did not show statistical significance according to T stage and Gleason score.
One of the limitations of our study was that the number of patients with positive PNIb was very small, so we were unable to estimate the value of PNIb as a prognostic factor. Another limitation was that detailed biopsy core data were not available for some patients. We recorded the presence or absence of PNIb in all cases, but did not quantify the extent or laterality of PNIb in some patients. Because the Gleason grading system has been changed, all the specimen slides were reviewed by a single pathologist. However, we had only the pathology reports for some patients who had undergone prostate biopsies at another hospital. Before 2005, we performed sextant prostate biopsies, whereas after 2005, extended multisite biopsies were performed. The rates of PNIb in the sextant biopsies and extended biopsies were 6.25% (4/64) and 3.37% (10/297), respectively. Therefore, we considered that the number of biopsy cores did not affect the detection of perineural invasion at prostate biopsy.
We found that PNIb had limited value in predicting PNIp. PNIp was significantly related to biologically aggressive tumor patterns but was not a prognostic factor for biochemical serum PSA recurrence or cancer-specific survival in patients with prostate cancer. The prognostic value of PNIp lacks statistical significance for biochemical serum PSA recurrence and cancer-specific survival.
References
1. Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009; 115:3379–3391. PMID: 19484787.
2. Marchesi F, Piemonti L, Montovani A, Allavena P. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev. 2010; 21:77–82. PMID: 20060768.

3. D'Amico AV, Wu Y, Chen MH, Nash M, Renshaw AA, Richie JP. Perineural invasion as a predictor of biochemical outcome following radical prostatectomy for select men with clinically localized prostate cancer. J Urol. 2001; 165:126–129. PMID: 11125380.
4. Freedland SJ, Csathy GS, Dorey F, Aronson WJ. Percent prostate needle biopsy tissue with cancer is more predictive of biochemical failure or adverse pathology after radical prostatectomy than prostate specific antigen or Gleason score. J Urol. 2002; 167:516–520. PMID: 11792909.

5. Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974; 111:58–64. PMID: 4813554.

6. Liebig C, Ayala G, Wilks J, Verstovsek G, Liu H, Agarwal N, et al. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol. 2009; 27:5131–5137. PMID: 19738119.

7. Ha HK, Yun CJ, Lee SS, Shin DG, Lee W, Lee ZZ, et al. Survival rates and related factors in men with hormone-refractory prostate cancer. Korean J Urol. 2009; 50:649–655.

8. Loeb S, Epstein JI, Humphreys EB, Walsh PC. Does perineural invasion on prostate biopsy predict adverse prostatectomy outcomes? BJU Int. 2010; 105:1510–1513. PMID: 19694710.

9. Ravery V, Boccon-Gibod LA, Dauge-Geffroy MC, Billebaud T, Delmas V, Meulemans A, et al. Systematic biopsies accurately predict extracapsular extension of prostate cancer and persistent/recurrent detectable PSA after radical prostatectomy. Urology. 1994; 44:371–376. PMID: 7521093.

10. Endrizzi J, Seay T. The relationship between early biochemical failure and perineural invasion in pathological T2 prostate cancer. BJU Int. 2000; 85:696–698. PMID: 10759668.

11. Ozcan F. Correlation of perineural invasion on radical prostatectomy specimens with other pathologic prognostic factors and PSA failure. Eur Urol. 2001; 40:308–312. PMID: 11684847.

12. Ng JC, Koch MO, Daggy JK, Cheng L. Perineural invasion in radical prostatectomy specimens: lack of prognostic significance. J Urol. 2004; 172:2249–2251. PMID: 15538241.

13. Quinn DI, Henshall SM, Brenner PC, Kooner R, Golovsky D, O'Neill GF, et al. Prognostic significance of preoperative factors in localized prostate carcinoma treated with radical prostatectomy: importance of percentage of biopsies that contain tumor and the presence of biopsy perineural invasion. Cancer. 2003; 97:1884–1893. PMID: 12673714.
14. Lee IH, Roberts R, Shah RB, Wojno KJ, Wei JT, Sandler HM. Perineural invasion is a marker for pathologically advanced disease in localized prostate cancer. Int J Radiat Oncol Biol Phys. 2007; 68:1059–1064. PMID: 17398032.

15. Jeon HG, Bae J, Yi JS, Hwang IS, Lee SE, Lee E. Perineural invasion is a prognostic factor for biochemical failure after radical prostatectomy. Int J Urol. 2009; 16:682–686. PMID: 19602004.

16. Stone NN, Stock RG, Parikh D, Yeghiayan P, Unger P. Perineural invasion and seminal vesicle involvement predict pelvic lymph node metastasis in men with localized carcinoma of the prostate. J Urol. 1998; 160:1722–1726. PMID: 9783940.

17. Miyake H, Sakai I, Harada K, Eto H, Hara I. Limited value of perineural invasion in radical prostatectomy specimens as a predictor of biochemical recurrence in Japanese men with clinically localized prostate cancer. Hinyokika Kiyo. 2005; 51:241–246. PMID: 15912782.
18. Merrilees AD, Bethwaite PB, Russell GL, Robinson RG, Delahunt B. Parameters of perineural invasion in radical prostatectomy specimens lack prognostic significance. Mod Pathol. 2008; 21:1095–1100. PMID: 18500264.

FIG. 1
Perineural invasion of prostate cancer cells. Prostate cancer cells grow along the nerve branch (red arrow) (H&E, ×400).
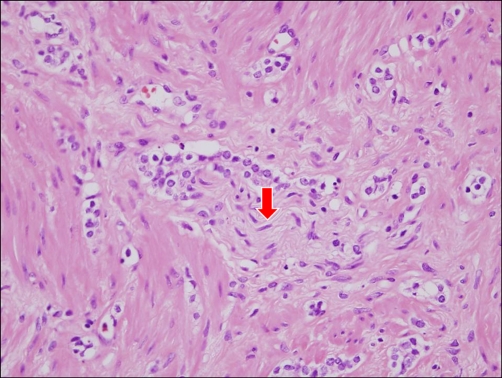
FIG. 2
Cumulative biochemical recurrence rate according to perineural invasion in a prostatectomy specimen (PNIp). The 5-year biochemical recurrence rate with and without PNIp was 0.808 and 0.850, respectively.

FIG. 3
Cumulative survival rate according to perineural invasion in a prostatectomy specimen (PNIp). The 5-year survival rate with and without PNIp was 0.914 and 0.777, respectively.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download