Abstract
Purpose
To evaluate the incidence of genitourinary mycoplasmas and the efficacy of antibiotics in women with overactive bladder (OAB) symptoms.
Materials and Methods
Women with OAB symptoms (micturition ≥8/24 hours and urgency ≥1/24 hours) for ≥3 months were screened for Mycoplasma hominis (M. hominis), Ureaplasma urealyticum (U. urealyticum), and Chlamydia trachomatis (C. trachomatis). Specimens from urethral and cervical vaginal swabs were examined for M. hominis and U. urealyticum by using the Mycoplasma IST2 kit and for C. trachomatis by using PCR. Women with positive results were treated with a 1 g dose of azithromycin. Persistent infection was treated with doxycycline. Changes in a 3-day bladder diary, Patient Perception of Bladder Condition (PPBC), and International Consultation on Incontinence Questionnaire-Female Lower Urinary Tract Symptoms (ICIQ-FLUTS) were evaluated 4 weeks after negative conversion. Patient satisfaction was assessed.
Results
Of 84 women screened, 42.8% were positive (U. urealyticum, 40.5%; M. hominis, 7.1%; C. trachomatis, 3.6%; two organisms, 8.3%). After treatment, 82.7% obtained negative conversion, and their median number of micturition episodes decreased from 10.6/24 hours to 8.1/24 hours (p=0.002). PPBC and domain scores of the ICIQ-FLUTS (filling and quality of life) significantly improved. About 87.5% women with negative conversion were satisfied with the treatment.
Overactive bladder (OAB) syndrome is described as "urgency, with or without urgency incontinence, usually with frequency and nocturia" by the International Continence Society (ICS) Terminology Committee [1]. However, a variety of medical conditions share the symptoms of OAB, so it is important to rule out these causes. Thus, the ICS Terminology Committee stated that the term OAB can be used only if there is no proven infection or other obvious pathology [1].
Urinary tract infection (UTI) is the most common condition that can cause OAB symptoms. The isolation of causative organisms through urine culture plays a crucial role in the diagnosis of UTI. However, even if ordinary bacteria are not cultured, there is some evidence to suggest that atypical organisms, such as mycoplasmas, may be associated with OAB symptoms [2-4].
Mycoplasmas are the simplest microorganisms and lack a cell wall, a feature largely responsible for their lack of a reaction to gram stain and their lack of susceptibility to drugs such as penicillin that interfere with cell wall synthesis [5]. Although scientists have isolated at least 17 species of mycoplasmas from humans, four types are responsible for clinically significant infections. These species are Mycoplasma pneumoniae, Mycoplasma hominis (M. hominis), Mycoplasma genitalium, and Ureaplasma species. These were generally viewed as commensals, although they usually manifest a predilection for particular host tissues, such as urogenital or respiratory tracts [6]. However, it is now being recognized that these organisms play a more important role in human infections than was previously thought. Their slow-growing, non-culturable nature enables them to establish chronic infections, resist the effects of antibiotics, and protect the organisms against immune system reactivity [7]. Accordingly, for patients presenting with chronic irritative urinary symptoms, especially those for whom ordinary cultures are negative, further tests can be indicated specifically for mycoplasmas.
Herein, we conducted a prospective study to estimate the incidence of genitourinary mycoplasma infections and to evaluate the efficacy of antibiotic treatment for these organisms in women with OAB symptoms.
This was a multicenter, prospective, single-arm study. The institutional review boards of the 4 study centers approved this study. Written informed consent was obtained from all participants.
Women with OAB symptoms for at least 3 months were screened. Inclusion criteria were women aged ≥20 and ≤80 years with micturition episodes ≥8/24 hours and urgency episodes ≥1/24 hours on a 3-day bladder diary. Urgency episodes were defined as the number of micturition episodes associated with a Urinary Sensation Scale rating ≥3 [8]. Exclusion criteria were a positive urine culture for bacteria using Gram stain, postvoid residual ≥150 ml, history of pelvic irradiation, neuropathic bladder, and evidence of chronic inflammation such as interstitial cystitis. Women who were pregnant or who had childbearing potential and did not use a reliable method of birth control during the study period were excluded. Patients who required concomitant administration of the following drugs or treatment modalities were also excluded: anti-cholinergics, alpha-blockers, tricyclic antidepressants, selective serotonin reuptake inhibitors, electrical or electromagnetic stimulation, and neuromodulation.
At the baseline visit, all participants were evaluated for their medical history, underwent a physical examination including pelvic examination, and completed the Patient's Perception of Bladder Condition (PPBC) [9] and International Consultation on Incontinence Questionnaire-Female Lower Urinary Tract Symptoms (ICIQ-FLUTS) [10]. Also, urethral and cervical vaginal swabs were obtained from all participants. The Mycoplasma IST2 kit (BioMerieux, Inc., Marcy-I'Etoile, France) was used for the culture, identification, and antibiotics susceptibility test for M. hominis and Ureaplasma urealyticum (U. urealyticum) [11]. Colony forming units ≥104/ml were regarded as a positive result for M. hominis and U. urealyticum infection. For the detection of Chlamydia trachomatis (C. trachomatis), polymerase chain reaction (PCR) was used, and a positive result was regarded as infection.
Four weeks after negative conversion, patients were evaluated by use of a 3-day bladder diary, the PPBC, the ICIQ-FLUTS, and Patient Perception of Treatment Benefit Questionnaire (PPTB) [12]. Primary endpoints were the percentage of positive cultures for M. hominis, U. urealyticum, and C. trachomatis, and the change in the number of micturition episodes after negative conversion. Secondary endpoints were the changes in the number of urgency episodes and changes on the PPBC, ICIQ-FLUTS, and PPTB. For patients with persistent infection after treatment, the bladder diary, PPBC, ICIQ-FLUTS, and PPTB were assessed 4 weeks after treatment.
Women with a positive result were initially treated with a 1 g dose of azithromycin. Two weeks after treatment, follow-up swab samples were taken from the previously positive site, either the urethra or the cervical vagina. Patients who had obtained negative conversion underwent no further treatment, whereas those with persistent infection were re-treated with 100 mg of doxycycline twice daily for 7 days, followed by additional swabs 2 weeks after the treatment. Identical treatment was given to the patients' sexual partners, and condom usage was recommended during the study period.
Potts et al showed that the difference in the mean number of micturition episodes was about -2.0/24 hours with a standard deviation of 3.5 after treatment of mycoplasmas in women with chronic urinary symptoms [2]. With a 0.05 significance level and 80% power to detect a difference in the number of micturition episodes of -2.0/24 hours, a sample size of 27 patients was needed to be positive for infection. Assuming a dropout rate of 20% and an incidence of mycoplasma infections in OAB patients of 20%, 160 women with OAB symptoms were scheduled to be screened.
Statistical analysis was performed by using the SPSS 17.0 (SPSS, Chicago, IL, USA) program for Windows® (Microsoft Corporation, Seattle, WA, USA). Wilcoxon signed rank test was used to compare nonparametric continuous variables. For all analyses, p<0.05 was considered statistically significant.
Of a total of 84 women screened, 42.8% (36/84) were found to be positive: 40.5% (34/84) for U. urealyticum, 7.1% (6/84) for M. hominis, and 3.6% (3/84) for C. trachomatis. About 8.3% (7/84) of women had 2 organisms: 7.1% (6/84) were positive for U. urealyticum and M. hominis and 1.2% (1/84) were positive for U. urealyticum and C. trachomatis. Overall, 7 of the 36 positive women were excluded: 5 because of having taken anti-cholinergics and 2 because of being lost to follow-up. Fig. 1 shows the participant flow. The demographic data and baseline characteristics of the 29 women are summarized in the Table 1. After treatment with antibiotics, 24 of 29 (82.7%) patients obtained negative conversion: 14 (48.3%) after the first treatment with azithromycin and 10 (34.5%) after the second treatment with doxycycline.
Among 24 women with negative conversion, the median number of micturition episodes per 24 hours was reduced from 10.6 (range, 8.0-19.3) to 8.1 (range, 4.0-20.7) (p=0.002). The median number of urgency episodes per 24 hours was reduced from 2.7 (range, 10.0-17.0) to 0.3 (range, 0-20.7) (p=0.097). In terms of PPBC, the median score was improved from 4.0 (range, 3.0-6.0) to 2.0 (range, 1.0-6.0) (p=0.001), and the scores for 58.3% (14/24) of the women were improved by at least 2 points. In terms of the ICIQ-FLUTS, the filling and quality of life domain scores improved significantly (Fig. 2). Twenty-one of 24 (87.5%) patients reported to have benefitted from the treatment and 9 (37.5%) of them reported "much benefit". Also 21 of 24 (87.5%) reported that they were satisfied with the treatment and 8 (33.3%) of them reported that they were "very satisfied".
For the 5 women with persistent infection after antibiotic treatment, the median number of micturition episodes per 24 hours was reduced from 9.3 (range, 8.0-14.3) to 8.3 (range, 6.0-15.0) (p=0.269), and the median number of urgency episodes was reduced from 3.3 (range, 1.0-9.3) to 0.0 (range, 0.0-8.0) (p=0.080). Changes on the PPBC and ICIQ-FLUTS were not statistically significant. Four of the five (80%) women reported having benefitted and one (20%) of them reported "much benefit" from the treatment. Four of five (80%) reported that they were satisfied with the treatment and none of them reported that they were "very satisfied".
Mycoplasma infection became a subject of interest in the early 1980s for its association with premature rupture of membranes, premature labor, endometritis, stillbirth, and neonatal respiratory distress syndrome. Several studies have attempted to implicate mycoplasma infection as a cause of chronic urinary symptoms [2-4]. Our results demonstrated that 42.8% of women with OAB symptoms were positive for mycoplasma and chlamydia infections.
The reported prevalence rates of mycoplasma infections are various. The wide ranges are due to differences in the method of isolation (e.g., culture, serological assay, PCR, antigen detection enzyme-linked immunoabsorbant assay), method of sample collection (e.g., urine, cervical/vaginal swab, vaginal discharge), geographical and economical factors, and social and sexual habits. Wave III of the National Longitudinal Study of Adolescent Health tested 14,322 young men and women for mycoplasma infection with urine specimens [13]. The prevalences of Mycoplasma genitalium (M. genitalium) and chlamydia were 1.0% and 4.2%, respectively. The prevalence was 11 times higher among respondents who reported living with a sexual partner, and increased by 10% for each additional sexual partner. Also, the prevalence was 7 times higher among Blacks [13]. On the contrary, a community-based study reported an extremely high rate of mycoplasma infection (>70% for M. hominis and >78% for U. urealyticum) in Papua New Guinea [14]. These rates are among the highest ever reported. The authors explained that both low socioeconomic status and experience with multiple sexual partners were associated with the high rates.
According to a retrospective comparative analysis of vaginal and endocervical cultures from 27,172 women visiting a genital tract diseases clinic, M. hominis was positive in 0.1% of symptomatic and 0.05% of asymptomatic women of childbearing age [15]. U. urealyticum was positive in 10.9% and 10.7%, respectively. This study reported that hormonal contraception and consistent condom use were protective factors for mycoplasma infections. In Central/West and North Africa, no steady partners, more than 1 partner in the previous 6 months, and intrauterine devices were risk factors. The presence of symptoms was not significantly related to the mycoplasma infections [15]. On the other hand, another study showed different prevalence rates according to the presence of symptoms [16]. Among symptomatic women in a sexually transmitted disease clinic, M. genitalium and C. trachomatis were positive in 6% (26/461) and 10% (45/465). However, the prevalence was 2% (1/59) and 0%, respectively, among women at a cancer screening center [16]. A Japanese study also showed 6.8% (8/117) of women with genital symptoms were positive for mycoplasma infections, compared with none of 80 women without symptoms [17]. Putting those various reports together, mycoplasmas have been isolated in up to 10% of women with genitourinary symptoms.
Our prevalence rate was higher than those reported in the above studies, but is similar to that of Potts et al who reported that 48% (23/48) of women with chronic urinary symptoms were positive for mycoplasmas (1 for M. hominis, 22 for U. urealyticum), although the inclusion criteria and the detection method differed from ours [2]. A recent study also revealed high prevalence rates of mycoplasma infections using the Mycoplasma IST2 kit, which was the same method that we used [4]. They reported that about 53% (81/153) of women with chronic voiding symptoms were positive for mycoplasmas (5 for M. hominis, 81 for U. urealyticum). The individual infection rate of M. hominis and U. urealyticum was 7.1% and 40.5%, respectively, in our study. M. hominis infection was always associated with U. urealyticum infection. In the latter study [4], M. hominis was also always detected in association with with U. urealyticum. High rates of co-infection of M. hominis with U. urealyticum were also reported in the above-mentioned prevalence studies. This can be explained by the fact that M. hominis and U. urealyticum are important opportunistic pathogens in the genitourinary tract.
After antimicrobial treatment, 24 of 29 (82.8%) infected women obtained negative conversion. For the 5 women with persistent infection, susceptible antibiotics were given on the basis of the result of a follow-up test after the last assessment. Four weeks after negative conversion, micturition frequency and scores on the symptom questionnaires were significantly improved, and the patient perception of treatment benefit and satisfaction was high. In other studies, micturition frequency and symptom severity were also improved after antimicrobial therapies [2,4]. However, we failed to prove significant improvement in urgency episodes and some domain scores of the ICIQ-FLUTS questionnaire. This might be due to the difference in patient characteristics. The other studies included women with a history of previous treatment for their symptoms. However, most of our patients (83%) had no history of treatment. Therefore, our patients might have had milder symptoms than in other studies. Apart from the patient characteristics, we used a voiding diary for quantitative assessment of urgency episodes and standardized questionnaires, which differed from the other studies, in which a symptom severity score was applied, with 0 points indicating mild or no symptoms and 3 points indicating severe symptoms. These might also be why the improvement in urgency episodes and some domain scores on the questionnaire was not significant.
Another study also suggested that urinary symptoms may be associated with unrecognized mycoplasma infection. Burkhard et al evaluated the efficacy of antimicrobial treatment (doxycycline) for urinary urgency, frequency, and chronic urethral and pelvic pain in women [3]. After treatment, 71% of the women became symptom-free or had a subjective decrease in symptoms [3]. Although causative organisms were not identified in most cases, the authors said that doxycycline was a broad-spectrum antibiotic that was effective against some of the most common bacterial causes of sexually transmitted diseases, such as mycoplasmas and chlamydia.
A limitation of our study is that there was no control group. Another limitation was that, though the improvement did not reach statistical significance, there was a considerable improvement in symptoms among the 5 women with persistent infection. We cannot explain this change, because there was no placebo group, but a placebo effect may be at least partially responsible. To confirm the causality of mycoplasma infections for OAB symptoms and the efficacy of antimicrobial treatment for the symptoms, randomized placebo-controlled trials are needed. Lastly, we enrolled patients who had only mild symptoms and no previous anticholinergic treatment. This may not reflect practice in real life, in which anticholinergics are the first-line treatment for OAB. Therefore, study with patients who have persistent OAB symptoms after anticholinergic medication would add more clinically relevant information on this subject.
The prevalence of genitourinary mycoplasma infection was high in women with OAB symptoms. After antimicrobial treatment, micturition frequency and symptom questionnaires were significantly improved, and the patient perception of treatment benefit and satisfaction were high. Diagnostic tests and treatment for genitourinary mycoplasmas would be beneficial for improving OAB symptoms in women before invasive and costly diagnostic tests and treatments are considered.
Figures and Tables
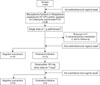 | FIG. 1Participant flow (n=84). a: identical treatment for the women's sexual partners, condom use during study period, b: after 2 weeks of treatment, from the previous infected site either urethra and/or cervical vagina. PCR: polymerase chain reaction. |
 | FIG. 2Changes in International Consultation on Incontinence Questionnaire-Female Lower Urinary Tract Symptoms after obtaining negative conversion (n=24). FS: filling sum, VS: voiding sum, IS: incontinence sum, Sex: sexual function, QoL: quality of
life. |
References
1. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002. 21:167–178.
2. Potts JM, Ward AM, Rackley RR. Association of chronic urinary symptoms in women and Ureaplasma urealyticum. Urology. 2000. 55:486–489.
3. Burkhard FC, Blick N, Hochreiter WW, Studer UE. Urinary urgency and frequency, and chronic urethral and/or pelvic pain in females. Can doxycycline help? J Urol. 2004. 172:232–235.
4. Baka S, Kouskouni E, Antonopoulou S, Sioutis D, Papakonstantinou M, Hassiakos D, et al. Prevalence of ureaplasma urealyticum and mycoplasma hominis in women with chronic urinary symptoms. Urology. 2009. 74:62–66.
5. Razin S. Balows A, Truper HG, Dworkin M, Harder W, Schleifer KH, editors. The genera mycoplasma, ureaplasma, acholeplasma, anaeroplasma, and asteroleplasma. The prokaryotes. 1991. 2nd ed. New York: Springer-Verlag;1937–1959.
6. Krause DC, Taylor-Robinson D. Maniloff RN, Mcelhaney LR, Finch JB, editors. Mycoplasmas which infect humans. Mycoplasmas: molecular biology and pathogenesis. 1992. Washington DC: American Society for Microbiology;417–444.
7. Dallo SF, Baseman JB. Intracellular DNA replication and long-term survival of pathogenic mycoplasmas. Microb Pathog. 2000. 29:301–309.
8. Brewster-Jordan JL, Guan Z, Green HL, Jumadilova Z, Coyne KS. Establishing the content validity of the Urinary Sensation Scale (USS). 2005. Washington, DC: International Society for Pharmacoeconomics and Outcomes Research.
9. Coyne KS, Matza LS, Kopp Z, Abrams P. The validation of the patient perception of bladder condition (PPBC): a single-item global measure for patients with overactive bladder. Eur Urol. 2006. 49:1079–1086.
10. Brookes ST, Donovan JL, Wright M, Jackson S, Abrams P. A scored form of the Bristol Female Lower Urinary Tract Symptoms questionnaire: data from a randomized controlled trial of surgery for women with stress incontinence. Am J Obstet Gynecol. 2004. 191:73–82.
11. Kilic D, Basar MM, Kaygusuz S, Yilmaz E, Basar H, Batislam E. Prevalence and treatment of Chlamydia trachomatis, Ureaplasma urealyticum, and Mycoplasma hominis in patients with non-gonococcal urethritis. Jpn J Infect Dis. 2004. 57:17–20.
12. Pleil AM, Coyne KS, Reese PR, Jumadilova Z, Rovner ES, Kelleher CJ. The validation of patient-rated global assessments of treatment benefit, satisfaction, and willingness to continue--the BSW. Value Health. 2005. 8:Suppl 1. S25–S34.
13. Manhart LE, Holmes KK, Hughes JP, Houston LS, Totten PA. Mycoplasma genitalium among young adults in the United States: an emerging sexually transmitted infection. Am J Public Health. 2007. 97:1118–1125.
14. Clegg A, Passey M, Yoannes M, Michael A. High rates of genital mycoplasma infection in the highlands of Papua New Guinea determined both by culture and by a commercial detection kit. J Clin Microbiol. 1997. 35:197–200.
15. Tibaldi C, Cappello N, Latino MA, Masuelli G, Marini S, Benedetto C. Vaginal and endocervical microorganisms in symptomatic and asymptomatic non-pregnant females: risk factors and rates of occurrence. Clin Microbiol Infect. 2009. 15:670–679.
16. Falk L, Fredlund H, Jensen JS. Signs and symptoms of urethritis and cervicitis among women with or without Mycoplasma genitalium or Chlamydia trachomatis infection. Sex Transm Infect. 2005. 81:73–78.
17. Uno M, Deguchi T, Komeda H, Hayasaki M, Iida M, Nagatani M, et al. Mycoplasma genitalium in the cervices of Japanese women. Sex Transm Dis. 1997. 24:284–286.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download