Abstract
Purpose
Intrarenal reflux (IRR) occurs in 3-10% of cases of total reflux and can lead to renal injury, which may eventually result in renal scarring. In this study, we evaluated the clinical importance of IRR detected by voiding cystourethrography and evaluated the relationship between IRR and renal scarring.
Materials and Methods
From January 2002 to May 2008, 50 patients who were diagnosed with vesicoureteral reflux (VUR) and showed IRR in voiding cystourethrography were enrolled. IRR was seen in 59 renal units in our enrolled patients. A 99mTc 2,3-dimercaptosuccinic acid (DMSA) renal scan was performed after VUR was detected in all cases. Nine patients were conservatively treated with prophylactic antibiotics, whereas 41 patients received an anti-reflux operation. A follow-up renal scan was performed after 3 to 6 months to check for any changes in renal scarring.
Results
The average patient age was 9.2 months (range, 1-42 months). Forty-nine patients were male; only one patient was female. The mean duration until surgery was 2.9 months. Generally, the IRR sites corresponded to the sites of photon defects on DMSA renal scans (76.3%). Furthermore, the photon defects on IRR sites tended to progress to renal scarring (65.2%). The rate of change in scarring was lower in the surgery group (47.1%) than in the prophylactic antibiotic group (75%).
Intrarenal reflux (IRR) involves intrarenal extension of the vesicoureteral reflux (VUR) into the tubular system of the kidney. This condition is diagnosed by voiding cystourethrography (VCUG). The incidence of IRR in patients who underwent VCUG for urinary tract infection has been reported to range from less than 1% to up to 10% [1,2]. IRR is the main cause of renal injury in children with VUR and may result in hypertension and reflux nephropathy with renal scars. IRR was first described by Brodeur et al in 1965 [3], and several IRR studies that investigated the relationship between IRR and reflux nephropathy were published in the 1970s. Over the past two decades, however, few additional studies investigating IRR have been published. There have been few reports about the result of operations in patients with IRR. Therefore, in this study, we investigated the relationship between IRR and photon defects by using 99mTc 2,3-dimercaptosuccinic acid scans (DMSA scans) and determined the propensity of IRR to progress to renal scarring based on follow-up DMSA scans according to treatment.
We performed a retrospective review of the medical records of 50 consecutive patients who were diagnosed with IRR on VCUG from January 2002 to May 2008. The average age of the patients at diagnosis was 9.17±9.19 months. Of these 50 patients, 49 (98%) were male; 49 (98%) presented with a febrile urinary tract infection and 1 (2%) was investigated because of prenatally diagnosed hydronephrosis.
DMSA scans were performed for all patients to evaluate renal damage within 1 week after VUR was diagnosed by VCUG. If photon defects were seen on the DMSA scan, we classified the position of the photon defect as the upper pole, mid pole, or lower pole. Four grades of photon defects were recognized: 0 (no photon defects), I (single photon defects), II (multiple photon defects), and III (relative uptake of renal radionuclide less than 20%). After patients were diagnosed with VUR, follow-up DMSA scans were performed every 3 to 6 months to evaluate whether the photon defect progressed to renal scarring or improved.
Nine patients were treated with prophylactic antibiotics only (medication group), whereas the remaining 41 patients had an anti-reflux operation (operation group). The average follow-up duration until the anti-reflux operation was 2.88±4.24 months.
In 50 patients, 18 renal units diagnosed with bilateral VUR only and not IRR were used as the control group.
We used SPSS software for Windows (version 15.0, SPSS, Chicago, USA) to process and analyze data. T-test was used to compare the grade of photon defect according to the presence of IRR. Pearson's chi-square test was used to compare differences between groups, and p<0.05 was considered statistically significant.
Most of the patients (82%) were younger than 1 year of age at the time of diagnosis (Table 1). VUR was demonstrated in 77 renal units. Of these renal units, IRR was demonstrated in 59.
In 59 renal units showing IRR, the VUR was grade II in 8 (14%), grade III in 10 (17%), grade IV (41%) in 24, and grade V (29%) in 17 renal units. Thus, the incidence of high-grade VUR (more than grade IV) was relatively high (70%) (Fig. 1).
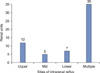
IRR on VCUG occurred in the upper pole in 12 cases, the mid pole in 5 cases, the lower pole in 7 cases, and multiple sites in 35 renal units (Fig. 2).
Of 59 renal units showing IRR, 46 renal units (80%) demonstrated photon defects on DMSA scans and 45 renal units (76.3%) had photon defects at the site coinciding with the site of IRR (Table 2). In renal units with IRR, the grade of the photon defect was 0 in 13 (22%), I in 12 (20.3%), II in 31 (52.6%), and III in 3 renal units (5.1%), indicating that photon defects were more severe in renal units with IRR than in control group renal units (p=0.001).
The incidence of scar formation in renal units showing photon defects at diagnosis was higher in the medication group (75% vs. 47%), but this difference was statistically insignificant (Table 3). In all 77 reflux renal units, we found that the incidence of scar formation was higher in renal units with IRR (50.8%: 30/59) than in those of the control group (22.2%: 4/18) (p=0.032) (Table 4).
Two important mechanisms play a major role in the pathogenesis of renal scarring in patients with VUR: renal scarring associated with the reflux of infected urine and congenital reflux nephropathy, which is characterized by the morphological features of renal dysplasia. Several reports on the relationship between renal scarring and IRR were published in the 1970s, but since then, few additional reports have been published. IRR is a condition that involves reflux of urine into collecting systems in the nephron, which can result in renal scarring with infected urine [4].
VCUG is the only practical diagnostic imaging modality available for IRR, but Darge et al reported that contrast-enhanced harmonic ultrasonography can facilitate more precise diagnosis of IRR than VCUG and can lower the chance of exposure to radiation at the same time [5]. The occurrence of IRR is closely related to the morphological features of renal papillae. Papillae with a convex shape have an oblique duct end that can produce a valvular effect that guards against backflow of urine into the medullary collecting ducts. Papillae with a concave architecture have their ducts directed at right angles, so the ends of ducts are still open when intrapelvic pressure increases, resulting in IRR [6]. In support of this, concave papillae are commonly distributed at polar calyces, where IRR is found preferentially [7]. In this study, the upper pole was the most frequent site of IRR; few cases of IRR were seen in the mid pole.
When urine is infected, it can result in endotoxin production, which in turn can result in inflammatory reactions, fibrosis of the renal parenchyma, and eventual renal scarring [7]. Renal scarring is slower in the absence of infection of the urine, but Ransley and Risdon reported that renal scarring can form in less than 4 weeks if the urine is infected [8]. Furthermore, these authors demonstrated that active treatment for bacteriuria using antibiotics decreased the frequency and degree of renal scarring. In pigs with VUR and even IRR, renal scarring occurs only when both VUR and bacteriuria are present. With sterile urine, there may be partial obstruction of the urethra resulting in a high voiding pressure and renal scarring [9,10].
However, some investigators have reported that reflux with sterile urine can result in renal scarring by urinary back pressure, the so-called "water hammer effect" [11,12]. Severe IRR with sterile urine leads to the destruction of the renal tubules, atrophic changes in the renal parenchyma, and thus renal scarring [13]. In neonates in particular, the kidney is vulnerable to pressure, and IRR can develop with only a slight increase in the intrapelvic pressure. Autopsy studies by Funston revealed that IRR may be created by pressures as low as 2 mmHg in normal neonates (younger than 1 month of age), whereas pressure of 20 mmHg may be needed to create IRR in a 1-year-old child. Furthermore, in children younger than 12 years, IRR occurs at 50 mmHg pressure [14]. This can explain why most cases of IRR are seen in neonates younger than 1 year of age; in our study, 82% of the patients were younger than 1 year of age at the time of diagnosis. Congenital obstructive lesions in the urethra (bulbous urethral ring, congenital urethral valve) were detected in 21 of 49 male patients, which suggests that intravesical pressure might be increased during voiding, leading to an increase in intrapelvic pressure.
The symptoms of urinary tract infection and pyelonephritis are often vague and nonspecific, resulting in delayed or inadequate treatment. Inflammatory responses in the kidney that can subsequently result in renal scar formation may therefore progress during this period without definite symptoms.
We thus compared the incidence of renal scarring between the operation group and the medication group with IRR. Although the distributions of reflux grade were not significantly different between the two groups, the incidence of renal scarring was higher in the medication group (75% vs. 47.2%). This implies that VUR with IRR should be treated more aggressively to prevent renal scarring.
Lim et al reported that the mean age of patients showing IRR on their VCUG was 4.76 months, and 82.4% of them were younger than 1 year of age [15]. In this study, most IRR was observed in neonates younger than 1 year (41/50), and a high grade of reflux (more than IV) was detected in 70% of IRR renal units, indicating that most patients had IRR combined with high-grade VUR. Photon defects visible on DMSA scans correlated well with the site of IRR on VCUG in 76.3% of cases. Among these cases, 65.2% (30/46) subsequently progressed to renal scarring, which was a higher incidence than that observed in the IRR-negative group (37.5%).
Because the distributions of reflux grade between the control group and the IRR group were not similar, and the number of subjects included in the control group was small, additional studies are required to confirm our findings.
Most cases of IRR were found in neonates younger than 1 year of age, and the sites of IRR were highly correlated with the sites of photon defects on DMSA renal scans and tended to progress to renal scars.
Therefore, more aggressive treatment should be considered in patients with combined VUR and IRR to prevent the formation of renal scars.
Figures and Tables
FIG. 1
Distributions of vesicoureteral reflux (VUR) grades according to the presence of intrarenal reflux (IRR).

TABLE 2
Incidence and grade of photon defects on DMSA scans in two groups of patient according to the presence of intrarenal reflux by renal units

References
1. Gotoh T, Asano Y, Nonomura K, Togashi M, Koyanagi T. Intrarenal reflux in children with vesicoureteral reflux. Nippon Hinyokika Gakkai Zasshi. 1991. 82:1480–1486.
2. Cremin BJ. Observations on vesico-ureteric reflux and intrarenal reflux: a review and survey of material. Clin Radiol. 1979. 30:607–621.
3. Brodeur AE, Goyer RA, Melick W. A potential hazard of barium cystography. Radiology. 1965. 85:1080–1084.
4. Bailey RR. The relationship of vesico-ureteric reflux to urinary tract infection and chronic pyelonephritis-reflux nephropathy. Clin Nephrol. 1973. 1:132–141.
5. Darge K, Trusen A, Gordjani N, Riedmiller H. Intrarenal reflux: diagnosis with contrast-enhanced harmonic US. Pediatr Radiol. 2003. 33:729–731.
6. Hodson CJ, Twohill SA. The time factor in the development of sterile renal scarring following high-pressure vesicoureteral reflux. Contrib Nephrol. 1984. 39:358–369.
7. Hannerz L, Wikstad I, Johansson L, Broberger O, Aperia A. Distribution of renal scars and intrarenal reflux in children with a past history of urinary tract infection. Acta Radiol. 1987. 28:443–446.
8. Ransley PG, Risdon RA. Reflux nephropathy: effects of antimicrobial therapy on the evolution of the early pyelonephritic scar. Kidney Int. 1981. 20:733–742.
9. Ransley PG, Risdon RA, Godley ML. High pressure sterile vesicoureteral reflux and renal scarring: an experimental study in the pig and minipig. Contrib Nephrol. 1984. 39:320–343.
10. Schulman SL, Snyder HM 3rd. Vesicoureteral reflux and reflux nephropathy in children. Curr Opin Pediatr. 1993. 5:191–197.
11. Rolleston GL, Shannon FT, Utley WL. Relationship of infantile vesicoureteric reflux to renal damage. Br Med J. 1970. 1:460–463.
12. Rolleston GL, Maling TM, Hodson CJ. Intrarenal reflux and the scarred kidney. Arch Dis Child. 1974. 49:531–539.
13. Hodson CJ, Maling TM, McManamon PJ, Lewis MG. The pathogenesis of reflux nephropathy (chronic atrophic pyelonephritis). Br J Radiol. 1975. 13:Suppl 13. 1–26.
14. Funston MR, Cremin BJ. Intrarenal reflux-papillary morphology and pressure relationships in children's necropsy kidneys. Br J Radiol. 1978. 51:665–670.
15. Lim BT, Lee HS, Pai KS. Clinical significance of intrarenal reflux in children with urinary tract infection. J Korean Soc Pediatr Nephrol. 2008. 12:186–193.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download