Abstract
The bone morphogenetic proteins (BMPs), as members of the transforming growth factor-β (TGF-β) superfamily, not only control bone formation, but also regulate multiple key steps during embryonic development and differentiation. Furthermore, BMPs play critical roles in maintaining the homeostasis of the cardiovascular, pulmonary, reproductive, urogenital, and nervous systems in adult life. Like all members of the TGF-β superfamily, BMP signaling is mediated through a heteromeric complex of type I and type II transmembrane serine/threonine kinase receptors. The subsequent signal transduction cascade includes either the canonical Smad-dependent or non-canonical Smad-independent pathways. Reflecting the critical function of BMPs, BMP signaling is tightly regulated at multiple steps by various mechanisms including extracellular endogenous antagonists, neutralizing antibodies/extracellular soluble receptor domains, small molecule inhibitors, cytoplasmic inhibitory Smads, and transcriptional co-repressors. Recently, dorsomorphin, the first small molecule inhibitor of BMP signaling, was identified and suggested as a useful tool for dissecting the mechanisms of signaling pathways and for developing novel therapeutics for diverse human diseases that are related to the BMP signaling pathways. In this article, we discuss various mechanisms involved in regulating BMP signaling pathways and their implications for urology.
Bone morphogenetic proteins (BMPs) are pleiotropic cytokines and members of the transforming growth factor-β (TGF-β) superfamily that include TGF-βs, activins, and mullerian inhibitory substance (MIS) [1]. Originally, BMPs were isolated from bone extract and were named for their effect on cartilage formation and de novo bone formation [2]. During the last two decades, over 20 different BMPs have been identified in both vertebrates and invertebrates [3].
More recently, detailed studies have revealed that BMPs not only control bone formation but also regulate embryonic development and differentiation [4-6]. Indeed, as with other members of the TGF-β superfamily, BMPs are important for gastrulation, mesoderm induction, organogenesis, proliferation, and apoptosis of multi-potent cells [7]. Besides the effect on embryonic development and differentiation, BMPs also play a critical role in homeostasis of the cardiovascular, pulmonary, reproductive, urogenital, and nervous systems in mature organisms [8]. Hence, BMPs have been linked to certain diseases such as primary pulmonary hypertension, fibrodysplasia ossificans progressiva, and juvenile polyposis syndrome [9-11]. Furthermore, recent reports in oncology revealed that BMPs are linked to carcinogenesis, including colorectal, ovarian, and lung cancers and melanoma [12-15]. Simultaneously, it has been reported that BMP-7 promotes brown adipogenesis. Specifically, Tseng et al reported that BMP-7 initiates the commitment of mesenchymal progenitor cells to a brown adipocyte linage and promotes the differentiation of brown preadipocytes [16]. Brown adipose tissue, unlike white adipose tissue, is essential in energy expenditure and may be a potential treatment for obesity [17].
Consistent with the diverse function of BMPs, BMP signaling is mediated through complex signal transduction pathways. Currently, over 20 known BMP ligands exert their effects through a heteromeric complex of both type I and type II transmembrane serine/threonine kinase receptors [18]. Following binding of the ligands, the combination of type I and type II receptors initiates a subsequent signal transduction cascade by phosphorylating Smads, which in turn rapidly move into the nucleus to modulate transcription [19]. Alternatively, BMP signaling involves Smad-independent pathways that include mitogen-activated protein kinase (MAPK) p38 [20]. Due to the critical role of BMPs, BMP signaling is tightly regulated at multiple steps throughout its signal transduction cascade. Among these regulatory mechanisms are endogenous inhibitors of BMPs such as noggin, which inhibit BMPs by sequestering the ligands [21,22]. In contrast, a small molecule inhibitor of BMPs, dorsomorphin, acts as a specific inhibitor of the BMP receptor type I. Because of this specificity, dorsomorphin may be a useful tool for dissecting the mechanisms of BMP signaling pathways in many biological processes as well as for developing novel therapeutics for various human diseases [23].
In this review, we summarize the current understanding of BMP signaling pathways and their regulatory mechanisms in depth, with a particular focus on the negative regulators, including endogenous and small molecule inhibitors.
The basic mechanism of BMP signaling has been well characterized by many investigators (Fig. 1). BMP signaling is transduced by a heteromeric complex of type I and type II transmembrane serine/threonine kinase receptors [18]. To date, three distinct type I receptors, activin receptor-like kinase 2 (ALK2), BMP type IA receptor (BMPR-IA/ALK3), and BMP type IB receptor (BMPR-IB/ALK6), have been identified [24]. Likewise, three type II receptors consisting of BMP type II receptor (BMPR-II), activin type IIA receptor (ActR-IIA), and activin type IIB receptor (ActR-IIB) have been described [25]. Both type I and type II receptors serve as components for the heteromeric, likely heterotetrameric, receptor complexes to which BMP ligands bind. Initially, the ligand binds to type II receptor, which then recruits type I receptor. Subsequently, type II receptor phosphorylates type I receptor, which in turn facilitates a subsequent signal transduction cascade by phosphorylating Smads, a group of intracellular mediators of BMP signaling [19].
Smads involved in BMP signaling are divided into three functional groups: 1) receptor-regulated Smads (R-Smads/Smad1, 5, and 8), 2) common-partner Smad (Co-Smad/Smad4), and 3) inhibitory Smads (I-Smads/Smad6 and 7) [19]. R-Smads directly interact with activated type I receptor and are phosphorylated. Activated R-Smads then form complexes with the lone Co-Smad. These R-Smads/Co-Smad complexes translocate into the nucleus to modulate the transcription of target genes [26]. In this Smad-dependent process, type I receptors appear to be key modulators of the specificity of BMP signaling pathways. For example, BMP-6 has a higher affinity for ALK2 receptor, through which Smad 1 and 5 are preferentially phosphorylated and activated. On the other hand, BMP-7 binds to all three type I receptors (ALK2, ALK3, and ALK 6), showing different patterns of affinity, and therefore all the R-Smads (Smad1, 5, and 8) are likely involved [15].
Although BMPs activate Smads for their signaling and it is clear that Smads are critical for BMP signaling pathways to modulate target gene transcription, alternative pathways different from the Smad pathway have been suggested. Like TGF-β and activin, BMP signaling can activate the mitogen-activated protein kinase (MAPK) p38 pathway independent of the Smads [20]. It has been proposed that ligand binding between BMPs and preformed heterodimeric receptor complexes leads to the Smad-dependent pathway, whereas induction of receptor complexes by the ligand results in the activation of the alternative MAPK p38 pathway [27,28].
Reflecting the critical roles of BMPs, BMP signaling is tightly regulated at multiple levels throughout its signaling cascades. Extracellular endogenous antagonists compose the first level of regulation, followed by cytoplasmic inhibitory Smads and transcriptional co-repressors (Table 1).
Various endogenous antagonists of BMPs, such as noggin, chordin, follistatin, gremlin, ectodin, cerberus, caronte, and sclerostin, are secreted into the extracellular space [29]. These secreted antagonists directly bind to BMP ligands, sequester active BMPs extracellularly, and prevent the binding of BMPs to the receptors [30]. The biological effects of these endogenous antagonists vary as they restrict and terminate BMP signaling.
Tight regulation of BMP signaling through the endogenous inhibitors is crucial during early embryogenesis [31]. Noggin and chordin induce neural tissue from ectoderm and dorsalize ventral mesoderm [32], whereas cerberus plays a critical role in the formation of the head [33]. Similarly, caronte regulates left-right symmetry [34], whereas limb development is controlled by noggin, chordin, follistatin, and gremlin [35-39]. The varying effects of these endogenous BMP antagonists are likely mediated by the differing affinity for BMP subtypes. For example, noggin has high affinity for BMP-2 and BMP-4 but low affinity for BMP-7; noggin also competes against gremlin and cerberus for BMP-2 [40,41].
The precise regulation of expression of these endogenous BMP antagonists remains to be elucidated. Nevertheless, a negative feedback look appears to be one important mechanism. For example, the increased expression of BMP-6 in prostate cancer cells has been associated with elevated levels of expression of noggin [42]. Similarly, in osteoprogenitor cells, expression of noggin is induced by BMP-2, 4, and 6 and the resulting increase in noggin decreases the bioactivity of these BMPs in a negative feedback loop [43].
Within the cytoplasm, the bioactivity of R-Smads (Smad1, 5, and 8) and Co-Smad (Smad4) is regulated by various inhibitors. These cytoplasmic antagonists of BMP signaling include inhibitory Smads (I-Smads/Smad6 and 7), Ski/SnoN, and Smurfs (Smad ubiquitination regulatory factors) [44,45]. I-Smads compete with R-Smads for the activated type I receptor and act as antagonists of R-Smad and Co-Smad signaling [46]. Between the two known I-Smads, Smad6 preferentially inhibits BMP signaling pathways, whereas Smad7 blocks TGF-β superfamily signaling pathways [46]. Mechanistically, I-Smads stably interact with the activated type I receptors, whereas R-Smads rapidly dissociate from these receptors. In addition, I-Smads, especially Smad6, compete with Co-Smad for the activated R-Smads. The physical interaction between R-Smads and Co-Smad is also regulated by Ski/SnoN; these proteins interfere with the process of complex formation between activated R-Smads and Co-Smad [47]. The last known endogenous mechanism of inhibiting BMP signaling involves the ubiquination-induced degradation via Smurfs [45].
In the context of the genitourinary system, BMPs are important for normal renal development. Although many BMPs are expressed during the organogenesis of the metanephric kidney [48], BMP-7 is expressed in both the ureteric epithelium and the mesenchyme throughout embryonic development [49]. BMP-7 knockout mice have bilateral renal agenesis, suggesting that BMP-7 is absolutely required for the proper formation of the kidneys [50]. On the other hand, the exact function of BMPs in the adult kidney is still largely unknown. Recently, Mitu and Hirschberg suggested a strong association between BMPs and chronic renal disease such as diabetic nephropathy [51]. While BMP-7 is heavily expressed by the podocytes, distal tubules, and collecting ducts in the adult kidney, expression of BMP-7 disappears in early fibrogenic renal diseases. Interestingly, the exogenous administration or transgenic overexpression of BMP-7 reduces renal fibrogenesis and better preserves nephron function and structural integrity [51].
In addition to the development of the genitourinary tract, BMPs have also been implicated in the cellular growth and progression of malignancy. In an effort to elucidate the molecular mechanisms of renal cell carcinogenesis, Kim et al investigated the potential role of BMP-6 and its receptors in renal cell carcinoma (RCC) cells [52]. They demonstrated that human RCC tissues frequently had decreased levels of expression of BMP receptor type II (BMP-RII), which was associated with insensitivity to the growth-inhibitory effect of BMP-6 in human RCC cells. These observations suggest that BMP-6 is a potent inhibitor of growth in human RCC cells and that abnormal expression of BMP-RII results in a resistance to the growth-inhibitory effect of BMP-6. Similarly, Basic-Jukic et al demonstrated higher BMP-6 mRNA expression levels in clear cell RCC tissue than in benign renal tissue [53]. In a more recent report, Blish et al reported that the BMP antagonist sclerostin domain-containing 1 (SOSTDC1) was down-regulated in clear cell RCC compared with normal kidney tissue [54]. In tissue culture, SOSTDC1 suppressed BMP-7-induced phosphorylation of Smad1, -5, and -8 and Wnt-3a signaling. These observations suggest that the restoration of SOSTDC1 expression may represent a novel therapeutic strategy for clear cell RCC because SOSTDC1 suppresses the proliferation of renal cell carcinoma cells.
Similar to the RCC studies, Kim et al evaluated the potential role of BMP receptors in bladder transitional cell carcinoma (TCC) cells and demonstrated that there was a significant association between the loss of BMP-RII expression and tumor grade [55]. Subsequently, they observed a decreased level of BMP-RII expression in a bladder TCC cell line, TSU-Pr1, and an enhanced resistance of the growth-inhibitory effect of BMP-6. These results suggest that the overexpression of BMP-RII may restore BMP signaling and decrease tumor growth in bladder TCC cells.
Compared to RCC and bladder TCC, the role of BMPs in prostate carcinogenesis has been extensively investigated during the last two decades. BMPs were initially implicated in the pathogenesis of osteoblastic metastasis-associated prostate adenocarcinoma, because BMP-7 was frequently overexpressed in the prostate and in metastatic bone lesions [56-58]. The precise role of BMP signaling in prostate cancer, though, has been controversial. Yuen et al reported that increased expression of BMP-6 and decreased expression of its inhibitors (noggin and sclerostin) promoted the metastatic progression of prostate cancer [59]. On the other hand, the expression of BMP-7 tended to be lower during the development and progression of prostate cancer, whereas its expression was highest in normal prostate glandular tissue [60]. Similarly, Buijs et al reported that BMP-7 can counteract the epithelial-to-mesenchymal transition process, suggesting that BMP-7 may be a potent inhibitor of prostate cancer bone metastasis in vivo [61]. With regard to the role of BMP membrane receptors (BMPRs) in prostate cancer, Kim et al observed that the loss of expression of BMPRs occurred more frequently in high-grade prostate cancer [62]. These observations, taken together, suggest that the loss of BMPRs contribute to prostate cancer progression and metastasis by releasing the transformed cells from the growth-inhibitory effect of BMPs.
Because overactive BMP signaling is often associated with various pathologic conditions, blocking BMPs may be an effective therapeutic approach. To this end, macromolecules such as neutralizing antibodies are currently being developed. These neutralizing antibodies contain a portion of the extracellular soluble receptors and recognize various BMP ligands as antigenic motifs and prevent the transduction of BMP signaling by neutralizing their activities through the antigen-antibody reaction [63].
Recently, Souza et al reported that the blockade of myostatin, a member of the TGF-β superfamily and a known regulator of muscle mass, resulted in significant muscle mass increases [64]. Specifically, adult mice were treated with anti-myostatin antibodies, a soluble form of the activin type IIB receptor (ActR-IIB), designated as ActRIIB-Fc (fragment crystallizable), and demonstrated a significant increase in muscle mass. Hence, myostatin is a potential therapeutic target for the treatment of various muscle-wasting diseases such as Duchenne muscular dystrophy (DMD), cachexia, and sarcopenia. Similarly, Natsume et al investigated the effect of extracellular domain type I receptor for BMP-2 and 4 by using a silkworm expression system and reported that this soluble form of BMP receptor (sBMPR) binds to BMPs tightly [65]. Indeed, sBMPR can act as a potent BMP antagonist by specifically binding to active forms of BMP-2 and 4.
In addition to the above macromolecules, small molecule inhibitors are chemical compounds that can act as selective inhibitory regulators of BMP signaling. To date, small molecule inhibitors have become a useful pharmacological tool for dissecting the mechanisms of signaling pathways in many biological processes associated with TGF-β signaling. For example, SB-431542 is a small molecule inhibitor that is a potent and specific inhibitor of TGF-β type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7 [66]. As such, SB-431542 has the potential to be a novel therapeutic agent for diverse human diseases in which TGF-β signaling has been implicated [67]. Similarly, Lee et al reported that IN-1130 is a relatively nontoxic and potent small molecule inhibitor of ALK4, ALK5, and ALK7 that inhibits TGF-β signaling as well as activin and nodal signaling [68]. In prostate cancer cell lines, IN-1130 exhibited an antitumor effect by blocking the immunosuppressive effects of the TGF-β signaling.
Small molecule inhibitors of BMP receptor kinases have recently been reported. Using an in vivo screening assay based on zebrafish, Yu et al identified a specific small molecule inhibitor of BMP signaling, dorsomorphin [23]. Further studies revealed that dorsomorphin inhibits BMP type I receptors ALK2, ALK3, and ALK6 and blocks subsequent phosphorylation of Smad1, 5, and 8 and target gene transcription [69]. Small molecule inhibitors, such as dorsomorphin, are relatively inexpensive, can penetrate many cell layers easily, and may be orally bioavailable. Thus, these inhibitors may be effective therapies for human pathologies associated with increased BMP signaling.
Reflecting their critical roles, BMPs are tightly regulated at multiple levels throughout their signal cascades. Extracellular endogenous antagonists, such as noggin and chordin, directly bind to BMP and sequester the activated ligands. In the cytoplasm, there are inhibitory Smads as well as Sno/Ski and Smurfs that regulate the R-Smad/Co-Smad interaction and R-Smad degradation, respectively.
Because the overexpression of BMPs has been implicated in a variety of human diseases including genitourinary malignancies, actively inhibiting BMP signaling may be an effective therapeutic approach in some patients. To this end, neutralizing antibodies and soluble receptor domains have been explored. Alternatively, dorsomorphin, the first small molecule inhibitor of BMP receptor type I, has been reported. As additional small molecule inhibitors are identified and characterized, it is likely that new therapeutics for various diseases will be discovered. Before initiating clinical trials that target BMP signaling pathways, the biology of BMPs and the regulatory mechanisms must be clarified.
Figures and Tables
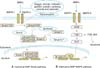 | FIG. 1BMP signal transduction. BMP signaling is transduced by both type I and type II transmembrane serine/threonine kinase receptors. BMPs bind to the heteromeric complex of type I and type II receptors. Subsequently, type II receptor phosphorylates type I receptor, which in turn facilitates phosphorylation of R-Smads (Smad1, 5, and 8). R-Smads directly interact with activated type I receptor and are released upon phosphorylation. Following the release from the receptor complex, R-Smads complex with Co-Smad (Smad4) and translocate into the nucleus to modulate the transcription of target genes (canonical BMP-Smad pathway). Independent of the Smads, BMPs may transduce signal via the MAPK p38 pathway. Endogenous mechanisms of BMP signaling inhibition include extracellular antagonists such as noggin and chordin, inhibitory Smads (Smad6 and 7), Sno/Ski, and Smurfs. The small molecule inhibitor dorsomorphin inhibits type I BMP receptors. →: stimuation, ┆---: inhibition, BMPs: bone morphogenetic protein, BMPR: BMP receptor, pSmad: phosphorylated Smad, Smurf: Smad ubiquitination regulatory factor, XIAP: human X-chromosome-linked inhibitor of apoptosis protein, TAK1: TGFβ-activated kinase 1, NLK: Nemo-like kinase, JNK: Jun kinase, MAPK: mitogen-activated protein kinase. |
References
1. Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998. 67:753–791.
2. Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988. 242:1528–1534.
3. Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002. 250:231–250.
4. Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004. 22:233–241.
5. Cunningham NS, Paralkar V, Reddi AH. Osteogenin and recombinant bone morphogenetic protein 2B are chemotactic for human monocytes and stimulate transforming growth factor beta 1 mRNA expression. Proc Natl Acad Sci U S A. 1992. 89:11740–11744.
6. Yamashita H, ten Dijke P, Huylebroeck D, Sampath TK, Andries M, Smith JC, et al. Osteogenic protein-1 binds to activin type II receptors and induces certain activin-like effects. J Cell Biol. 1995. 130:217–226.
7. Zhao GQ. Consequences of knocking out BMP signaling in the mouse. Genesis. 2003. 35:43–56.
8. Goumans MJ, Mummery C. Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int J Dev Biol. 2000. 44:253–265.
9. Billings PC, Fiori JL, Bentwood JL, O'Connell MP, Jiao X, Nussbaum B, et al. Dysregulated BMP signaling and enhanced osteogenic differentiation of connective tissue progenitor cells from patients with fibrodysplasia ossificans progressiva (FOP). J Bone Miner Res. 2008. 23:305–313.
10. Haramis AP, Begthel H, van den Born M, van Es J, Jonkheer S, Offerhaus GJ, et al. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004. 303:1684–1686.
11. Yu PB, Beppu H, Kawai N, Li E, Bloch KD. Bone morphogenetic protein (BMP) type II receptor deletion reveals BMP ligand-specific gain of signaling in pulmonary artery smooth muscle cells. J Biol Chem. 2005. 280:24443–24450.
12. Hardwick JC, Kodach LL, Offerhaus GJ, van den Brink GR. Bone morphogenetic protein signalling in colorectal cancer. Nat Rev Cancer. 2008. 8:806–812.
13. Theriault BL, Shepherd TG, Mujoomdar ML, Nachtigal MW. BMP4 induces EMT and Rho GTPase activation in human ovarian cancer cells. Carcinogenesis. 2007. 28:1153–1162.
14. Kraunz KS, Nelson HH, Liu M, Wiencke JK, Kelsey KT. Interaction between the bone morphogenetic proteins and Ras/MAP-kinase signalling pathways in lung cancer. Br J Cancer. 2005. 93:949–952.
15. Hsu MY, Rovinsky S, Penmatcha S, Herlyn M, Muirhead D. Bone morphogenetic proteins in melanoma: angel or devil? Cancer Metastasis Rev. 2005. 24:251–263.
16. Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008. 454:1000–1004.
17. Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007. 131:242–256.
18. Sebald W, Nickel J, Zhang JL, Mueller TD. Molecular recognition in bone morphogenetic protein (BMP)/receptor interaction. Biol Chem. 2004. 385:697–710.
19. Miyazono K, Kusanagi K, Inoue H. Divergence and convergence of TGF-beta/BMP signaling. J Cell Physiol. 2001. 187:265–276.
20. Nohe A, Keating E, Knaus P, Petersen NO. Signal transduction of bone morphogenetic protein receptors. Cell Signal. 2004. 16:291–299.
21. Ito H, Akiyama H, Shigeno C, Nakamura T. Noggin and bone morphogenetic protein-4 coordinately regulate the progression of chondrogenic differentiation in mouse clonal EC cells, ATDC5. Biochem Biophys Res Commun. 1999. 260:240–244.
22. Zhu W, Kim J, Cheng C, Rawlins BA, Boachie-Adjei O, Crystal RG, et al. Noggin regulation of bone morphogenetic protein (BMP) 2/7 heterodimer activity in vitro. Bone. 2006. 39:61–71.
23. Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008. 4:33–41.
24. ten Dijke P, Yamashita H, Ichijo H, Franzen P, Laiho M, Miyazono K, et al. Characterization of type I receptors for transforming growth factor-beta and activin. Science. 1994. 264:101–104.
25. Rosenzweig BL, Imamura T, Okadome T, Cox GN, Yamashita H, ten Dijke P, et al. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci U S A. 1995. 92:7632–7636.
26. Derynck R, Zhang Y, Feng XH. Smads: transcriptional activators of TGF-beta responses. Cell. 1998. 95:737–740.
27. Nohe A, Hassel S, Ehrlich M, Neubauer F, Sebald W, Henis YI, et al. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J Biol Chem. 2002. 277:5330–5338.
28. Hassel S, Schmitt S, Hartung A, Roth M, Nohe A, Petersen N, et al. Initiation of Smad-dependent and Smad-independent signaling via distinct BMP-receptor complexes. J Bone Joint Surg Am. 2003. 85:A Suppl 3. 44–51.
29. Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003. 24:218–235.
30. Larrain J, Bachiller D, Lu B, Agius E, Piccolo S, De Robertis EM. BMP-binding modules in chordin: a model for signalling regulation in the extracellular space. Development. 2000. 127:821–830.
31. Miyazono K. Positive and negative regulation of TGF-beta signaling. J Cell Sci. 2000. 113:1101–1109.
32. Stottmann RW, Anderson RM, Klingensmith J. The BMP antagonists Chordin and Noggin have essential but redundant roles in mouse mandibular outgrowth. Dev Biol. 2001. 240:457–473.
33. Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, et al. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999. 397:707–710.
34. Yokouchi Y, Vogan KJ, Pearse RV 2nd, Tabin CJ. Antagonistic signaling by Caronte, a novel Cerberus-related gene, establishes left-right asymmetric gene expression. Cell. 1999. 98:573–583.
35. Capdevila J, Johnson RL. Endogenous and ectopic expression of noggin suggests a conserved mechanism for regulation of BMP function during limb and somite patterning. Dev Biol. 1998. 197:205–217.
36. Zhang D, Ferguson CM, O'Keefe RJ, Puzas JE, Rosier RN, Reynolds PR. A role for the BMP antagonist chordin in endochondral ossification. J Bone Miner Res. 2002. 17:293–300.
37. Tardif G, Pelletier JP, Boileau C, Martel-Pelletier J. The BMP antagonists follistatin and gremlin in normal and early osteoarthritic cartilage: an immunohistochemical study. Osteoarthritis Cartilage. 2009. 17:263–270.
38. Michos O, Panman L, Vintersten K, Beier K, Zeller R, Zuniga A. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development. 2004. 131:3401–3410.
39. Khokha MK, Hsu D, Brunet LJ, Dionne MS, Harland RM. Gremlin is the BMP antagonist required for maintenance of Shh and Fgf signals during limb patterning. Nat Genet. 2003. 34:303–307.
40. Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996. 86:599–606.
41. Hsu DR, Economides AN, Wang X, Eimon PM, Harland RM. The Xenopus dorsalizing factor Gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol Cell. 1998. 1:673–683.
42. Haudenschild DR, Palmer SM, Moseley TA, You Z, Reddi AH. Bone morphogenetic protein (BMP)-6 signaling and BMP antagonist noggin in prostate cancer. Cancer Res. 2004. 64:8276–8284.
43. Tezval M, Tezval H, Dresing K, Stuermer EK, Blaschke M, Stuermer KM, et al. Differentiation dependent expression of urocortin's mRNA and peptide in human osteoprogenitor cells: influence of BMP-2, TGF-beta-1 and dexamethasone. J Mol Histol. 2009. 40:331–341.
44. Hanyu A, Ishidou Y, Ebisawa T, Shimanuki T, Imamura T, Miyazono K. The N domain of Smad7 is essential for specific inhibition of transforming growth factor-beta signaling. J Cell Biol. 2001. 155:1017–1027.
45. Zhu HT, Kavsak P, Abdollah S, Wrana JL, Thomsen GH. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature. 1999. 400:687–693.
46. Hata A, Lagna G, Massague J, Hemmati-Brivanlou A. Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Gene Dev. 1998. 12:186–197.
47. Zwijsen A, Verschueren K, Huylebroeck D. New intracellular components of bone morphogenetic protein/Smad signaling cascades. FEBS Lett. 2003. 546:133–139.
48. Dudley AT, Robertson EJ. Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev Dyn. 1997. 208:349–362.
49. Godin RE, Robertson EJ, Dudley AT. Role of BMP family members during kidney development. Int J Dev Biol. 1999. 43:405–411.
50. Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995. 9:2795–2807.
51. Mitu G, Hirschberg R. Bone morphogenetic protein-7 (BMP7) in chronic kidney disease. Front Biosci. 2008. 13:4726–4739.
52. Kim IY, Lee DH, Lee DK, Kim BC, Kim HT, Leach FS, et al. Decreased expression of bone morphogenetic protein (BMP) receptor type II correlates with insensitivity to BMP-6 in human renal cell carcinoma cells. Clin Cancer Res. 2003. 9:6046–6051.
53. Basic-Jukic N, Radic-Antolic M, Hudolin T, Coric M, Zadro R, Pasini J, et al. Immunolocalization and mRNA expression of bone morphogenetic protein-6 in human clear cell renal carcinoma. Kidney Blood Press Res. 2009. 32:445–450.
54. Blish KR, Wang W, Willingham MC, Du W, Birse CE, Krishnan SR, et al. A human bone morphogenetic protein antagonist is down-regulated in renal cancer. Mol Biol Cell. 2008. 19:457–464.
55. Kim IY, Lee DH, Lee DK, Kim WJ, Kim MM, Morton RA, et al. Restoration of bone morphogenetic protein receptor type II expression leads to a decreased rate of tumor growth in bladder transitional cell carcinoma cell line TSU-Pr1. Cancer Res. 2004. 64:7355–7360.
56. Bentley H, Hamdy FC, Hart KA, Seid JM, Williams JL, Johnstone D, et al. Expression of bone morphogenetic proteins in human prostatic adenocarcinoma and benign prostatic hyperplasia. Br J Cancer. 1992. 66:1159–1163.
57. Hamdy FC, Autzen P, Robinson MC, Horne CH, Neal DE, Robson CN. Immunolocalization and messenger RNA expression of bone morphogenetic protein-6 in human benign and malignant prostatic tissue. Cancer Res. 1997. 57:4427–4431.
58. Masuda H, Fukabori Y, Nakano K, Takezawa Y, CSuzuki T, Yamanaka H. Increased expression of bone morphogenetic protein-7 in bone metastatic prostate cancer. Prostate. 2003. 54:268–274.
59. Yuen HF, Chan YP, Cheung WL, Wong YC, Wang X, Chan KW. The prognostic significance of BMP-6 signaling in prostate cancer. Mod Pathol. 2008. 21:1436–1443.
60. Masuda H, Fukabori Y, Nakano K, Shimizu N, Yamanaka H. Expression of bone morphogenetic protein-7 (BMP-7) in human prostate. Prostate. 2004. 59:101–106.
61. Buijs JT, Rentsch CA, van der Horst G, van Overveld PG, Wetterwald A, Schwaninger R, et al. BMP7, a putative regulator of epithelial homeostasis in the human prostate, is a potent inhibitor of prostate cancer bone metastasis in vivo. Am J Pathol. 2007. 171:1047–1057.
62. Kim IY, Lee DH, Ahn HJ, Tokunaga H, Song W, Devereaux LM, et al. Expression of bone morphogenetic protein receptors type-IA, -IB and -II correlates with tumor grade in human prostate cancer tissues. Cancer Res. 2000. 60:2840–2844.
63. Sugimoto H, Yang C, LeBleu VS, Soubasakos MA, Giraldo M, Zeisberg M, et al. BMP-7 functions as a novel hormone to facilitate liver regeneration. FASEB J. 2007. 21:256–264.
64. Souza TA, Chen X, Guo Y, Sava P, Zhang J, Hill JJ, et al. Proteomic identification and functional validation of activins and bone morphogenetic protein 11 as candidate novel muscle mass regulators. Mol Endocrinol. 2008. 22:2689–2702.
65. Natsume T, Tomita S, Iemura S, Kinto N, Yamaguchi A, Ueno N. Interaction between soluble type I receptor for bone morphogenetic protein and bone morphogenetic protein-4. J Biol Chem. 1997. 272:11535–11540.
66. Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002. 62:65–74.
67. Halder SK, Beauchamp RD, Datta PK. A specific inhibitor of TGF-beta receptor kinase, SB-431542, as a potent antitumor agent for human cancers. Neoplasia. 2005. 7:509–521.
68. Lee GT, Hong JH, Mueller TJ, Watson JA, Kwak C, Sheen YY, et al. Effect of IN-1130, a small molecule inhibitor of transforming growth factor-beta type I receptor/activin receptor-like kinase-5, on prostate cancer cells. J Urol. 2008. 180:2660–2667.
69. Hao J, Daleo MA, Murphy CK, Yu PB, Ho JN, Hu J, et al. Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells. PLoS One. 2008. 3:e2904.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download