Abstract
An extratesticular scrotal epidermoid cyst is a relatively very rare condition, and an epidermoid cyst arising from the spermatic cord area is extremely rare. We report a case of multiple epidermoid cysts arising from the extratesticular scrotum, spermatic cord, and lower extremities. To our best knowledge, concomitant occurrence of these lesions has not been reported previously in the literature.
Epidermoid cysts are the most common benign epithelial cysts. They are well encapsulated cysts and histologically characterized by a cystic lining of stratified squamous epithelial cells and no cutaneous adnexal structures in the stromal tissue [1]. Extratesticular, scrotal epidermoid cysts are relatively rare in contrast to intratesticular ones, which are the most common benign testicular neoplasm (1% of all testicular masses) [2]. Epidermoid cysts of the spermatic cord area are extremely rare and only 4 cases have been previously reported in the literature [3,4]. To our knowledge, this is the first reported case of an epidermoid cyst occurring on the spermatic cord in conjunction with ones in the paratesticular and lower extremities simultaneously.
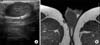
A 39-year-old male patient presented with a slow-growing, painless, scrotal enlargement with a soft consistency growing over a 2-year duration. Recently, another 2 cm sized, ovoid lesion had been newly palpated on the contralateral spermatic cord area. His medical history revealed no evidence of previous urinary tract infection, tuberculosis, abdomino-pelvic surgery, or scrotal trauma. On physical examination, non-tender, elastic, freely movable masses were palpated at the lower right scrotal base and contralateral inguinal area, which was adjacent to the left spermatic cord. The overlying skin showed no abnormality and no gynecomastia was observed. Testicular tumor markers (alpha-fetoprotein, beta-chorionic gonadotropin, and lactate dehydrogenase) and hormones were within the normal limits. Gray scale ultrasonography with high frequency transducer showed an oval-shaped, homogenous, hypoechoic, well-encapsulated solid mass with scattered internal thin septa-like structures without evidence of calcification or communication with the testis parenchyma in the caudal scrotum. The tumor on the spermatic cord area showed a very similar ultrasonographic appearance (Fig. 1A). The color flow Doppler sonography showed no intratumoral vascular signals, suggesting that they were not typical solid masses. On the pelvic MRI, the masses showed homogenous, low signal intensity on T1-weighted images and intermediate signal intensity on T2-weighted images. Furthermore, they were located within the subcutaneous layer and sharply demarcated from surrounding structures. Gadolinium-enhanced T1-weighted images showed peripheral rim enhancement without significant internal enhancement, suggestive of a cystic structure encapsulated within a thin fibrous capsule (Fig. 1B). An incidental cystic mass was also detected at the medial portion of the right upper thigh.
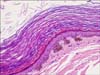
A complete surgical excision was performed. The scrotal mass was a 5×5 cm sized cystic mass without any connection with the adjacent testis, filled compactly with a brownish soft, pastelike material on gross inspection. The cystic wall showed no solid portion without evidence of rupture or secondary foreign body-type granulomatous reaction or abscess (Fig. 2). The inguinal mass was easily separated from the layers of the spermatic cord, and it also contained brownish sebaceous material in the cyst. The mass on the upper thigh was also removed. There were no teratomatous components or adnexal structures such as sebaceous glands, hair follicles, or sweat glands within the cystic lumen. Microscopic evaluation demonstrated a cystic structure, lined by keratinized squamous epithelium with laminated keratin materials in the lumen (Fig. 3).
An epidermoid cyst is the most common benign simple epithelial cyst without malignant potential, most often found on the scalp, face, trunk, and back. It is characterized histologically by a cyst lining of stratified squamous cells and loosely packed lamellae of keratin debris, cholesterol, and water without teratomatous elements or skin appendages in the stromal tissue [1]. The histogenesis of the epidermoid cyst is not precisely known, but there are different theories about the embryonic origin of this lesion: (i) they arise from ectopic cutaneous tissue due to dislocation of tissue into a neighboring area; (ii) they are end results of monolayer teratoma from germ cells; (iii) they occur due to traumatic implantation of epidermal tissue into the dermis and subcutis [3]. However, in the case of the extratesticular scrotal epidermoid cyst, they are believed to be an abnormal closure or associated degenerative process of the median raphe and urethral groove [4,5].
Scrotal ultrasonography is usually the first and the most important imaging modality to investigate scrotal and inguinal pathologic conditions, and it can reliably differentiate extratesticular from intratesticular ones, as well as cystic from solid ones, with very high accuracy. The sonographic appearance of an epidermal cyst varies from an anechoic lesion to a hyperechoic, solid appearing mass, depending on its content. Although there are no specific pathognomonic findings to diagnose the epidermoid cysts accurately, they have most often appeared with well demarcated hypoechoic masses with multiple scattered reflectors from the keratinous debris and posterior sound enhancement without internal vascular signals on the color flow Doppler sonography. Some cases show specific manifestations of an onionskin appearance of concentric, swirling rings of alternating dense echogenicity and sonolucency [6]. This is pathologically well correlated with alternating layers of laminating keratin debris and loosely scattered desquamated squamous cells. In other cases, the keratinized debris primarily collected centrally, producing a centrally dense echogenic focus that has been described as having the appearance of a "target" of a "bull's-eye" as the keratinized debris primarily collects centrally, producing a central echogenic focus [7,8]. Dynamic-enhanced MR imaging is very precise in showing the pelvis structures and frequently gives additional useful information to help to characterize the mass. Epidermoid cysts have usually shown as thin-walled cysts with hypointensity on T1-weighted images and hyperintensity on T2-weighted images. Epidermoid cysts have usually shown internal heterogenous intensity on T1- and T2-weighted images due to inner dense keratinous materials. Gadolinium-enhanced images usually show no enhancement with or without a peripheral enhancement of the cystic wall [1,2].
Tumors of the spermatic cord and paratesticular tissue are uncommon and mostly benign. The most common benign paratesticular tumor is mesothelioma of the epididymis, closely followed by lipoma, leiomyoma, fibroma, and hemangioma. Primary malignant epithelial tumors of the paratesticular region are exceedingly rare and are mainly known to be rhabdomyosarcoma [9].
Complete local excision is considered the treatment of choice for an epidermoid cyst because there have been no reported cases of malignant transformation or distant metastasis in the literature, in contrast with dermoid cysts, which have malignant potential. In addition, cross-sectional imaging studies such as scrotal sonography and MRI are unable to differentiate a benign from a malignant mass accurately, so complete local excision with intraoperative frozen section biopsy should be done when malignancy is suspected.
Figures and Tables
FIG. 1
(A) Well demarcated, homogenous, hypoechoic mass closely adjacent to the spermatic cord. (B) Gadolinium-enhanced T1-weighted magnetic resonance image showing subcutaneous masses in the scrotum and inner thigh with only peripheral wall enhancement.
References
1. Langer JE, Ramchandani P, Siegelman ES, Banner MP. Epidermoid cysts of the testicle: sonographic and MR imaging features. AJR Am J Roentgenol. 1999. 173:1295–1299.
2. Cho JH, Chang JC, Park BH, Lee JG, Son CH. Sonographic and MR imaging findings of testicular epidermoid cysts. AJR Am J Roentgenol. 2002. 178:743–748.
3. Tanaka T, Yasumoto R, Kawano M. Epidermoid cyst arising from the spermatic cord area. Int J Urol. 2000. 7:277–279.
4. Katergiannakis V, Lagoudianakis EE, Markogiannakis H, Manouras A. Huge epidermoid cyst of the spermatic cord in an adult patient. Int J Urol. 2006. 13:95–97.
5. Picanco-Neto JM, Lipay MA, D'Avila CL, Verona CB, Zerati-Filho M. Intrascrotal epidermoid cyst with extension to the rectum wall: a case report. J Pediatr Surg. 1997. 32:766–767.
6. Lee HS, Joo KB, Song HT, Kim YS, Park DW, Park CK, et al. Relationship between sonographic and pathologic findings in epidermal inclusion cysts. J Clin Ultrasound. 2001. 29:374–383.
7. Wegner HE, Herbst H, Dieckmann KP. Paratesticular epidermoid cyst and ipsilateral spermatic cord dermoid cyst: case report and discussion of pathogenesis, diagnosis and treatment. J Urol. 1994. 152:2101–2103.
8. Malvica RP. Epidermoid cyst of the testicle: an unusual sonographic finding. AJR Am J Roentgenol. 1993. 160:1047–1048.
9. Lioe TF, Biggart JD. Tumours of the spermatic cord and paratesticular tissue. A clinicopathological study. Br J Urol. 1993. 71:600–606.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download