Abstract
Urachal adenocarcinomas are very rare and about one third of these neoplasms arise in urachal remnants. To demonstrate the origin of the urachal adenocarcinoma is not easy, but it is very important for managing patient care. We report on a 35-year-old man who complained of a palpable mass in the periumbilical area. The mass was incidentally identified 10 days earlier. Computed tomography revealed a well-defined enhancing mass with internal calcification and septation abutting on the dome of the urinary bladder. The clinical diagnosis was urachal cancer, which seemed to invade the urinary bladder. Thus, we performed mass excision and partial resection of the bladder. Histopathologically, the mass was diagnosed as mucinous cystadenocarcinoma originating from urachal remnants that showed an unusual expression of alpha-methylacyl-coenzyme A racemase (AMACR). To our knowledge, this report is the first case of AMACR-expressing urachal adenocarcinoma arising in the abdominal wall.
Urachal adenocarcinomas are an uncommon malignancy accounting for 0.17-0.34% of all bladder cancers and 20-39% for adenocarcinomas of the bladder [1]. Diagnosis of urachal adenocarcinoma often requires distinguishing it from primary bladder cancer and metastasis from other sites. Although distinction between them is important in clinical management, there have been few studies about this issue and no reliable markers have been described [2,3]. Alpha-methylacyl-coenzyme A racemase (AMACR) is an enzyme involved in β-oxidation of branched-chain fatty acids in mitochondria and peroxisomes, and it is frequently expressed in prostatic carcinomas and occasionally in other types of carcinomas [4-6]. We report a case of urachal adenocarcinoma arising in the abdominal wall with expression of AMACR and review the related literature.
A 35-year-old man had presented with a periumbilical mass 10 days earlier. On physical examination, the abdomen was fully protruded, and the mass was palpable. Laboratory tests of blood and urinary analysis were within normal limits. Computed tomography (CT) revealed a well-circumscribed, multilocular cystic mass along the midline of the lower abdomen, measuring 9 cm in its maximum diameter. The mass was located adjacent to the dome of the urinary bladder. Punctate, annular, curvilinear and peripheral calcifications were demonstrated in the cystic wall and septa (Fig. 1). The anterior portion of this mass was surrounded by the areolar fat layer of the prevesical space of Retzius and this location strongly suggested its urachal origin. There was no evidence of any other solid organ mass or metastatic lesion by CT.
In cystoscopic findings, there were no abnormal mucosal lesions. Laparotomy was carried out, and an about 10 cm sized hard mass was identified. The mass was cystic, encapsulated and extraperitoneally located in the prevesical space, and it was attached to the dome of the urinary bladder and continued to the umbilicus with a urachal remnant. The cystic mass and urachal remnant were resected and partial resection of the bladder was performed, including a cuff of the bladder as well. Grossly, the resected bladder margin and inner mucosa were clear without invasion, and we found no evidence of peritoneal implants or pseudomyxoma peritonei in the intraperitoneal space.
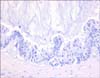
Microscopically, the tumor was composed of stratified columnar epithelia that lined a cyst. The tumor cells showed oval to round nuclei and abundant cytoplasm (Fig. 2). Mitotic figures were frequently identified. Acellular mucin dissected through the connective tissue mimicking invasion, which resulted in diffuse inflammatory responses (Fig. 3). By immunohistochemistry, the tumor cells were positive for cytokeratin 7 (weak), cytokeratin 20, carcinoembryonic antigen (CEA) and AMACR, and negative for prostate-specific antigen (PSA) and high molecular weight cytokeratin (Fig. 4). The final diagnosis was mucinous cystadenocarcinoma arising in the urachal remnant of the abdominal wall. The patient is alive and well with no evidence of recurrence clinically or radiographically at 12 months after treatment.
Urachal adenocarcinomas are rare, representing less than 0.5% of all bladder cancers and 20-39% of primary adenocarcinomas of the bladder [1]. Because urachal remnant diseases are uncommon and manifest with nonspecific abdominal or urinary signs or symptoms, a definitive presurgical diagnosis is not easy [7]. Urachal neoplasms follow variable clinical courses. Some cases are associated with early local invasion, so they have a poor prognosis. On the other hand, there are indolent cases of urachal tumours confined for a long time without local invasion or distant metastases [8]. Unfortunately, it is not easy to distinguish urachal adenocarcinoma from other origins of adenocarcinoma, but distinction between them is important in determining an appropriate therapy and assessing prognosis. Although the normal urachus is most commonly lined by transitional epithelium, urachal carcinomas predominantly manifest as adenocarcinomas, which are lined by mucus-secreting epithelial cells resembling those of the colorectum, probably due to metaplasia of the urachal mucosa into columnar epithelium followed by malignant transformation [9]. Therefore, a biologic marker for confirming the origin of the urachal adenocarcinoma is needed.
Several studies have differentiated among bladder, urachal, and metastatic adenocarcinoma. Torenbeek et al showed that CEA, PSA, OC125, and vimentin can be helpful in differentiating primary adenocarcinomas of the urinary bladder from secondary involvement by adenocarcinomas originating from surrounding organs [2]. CEA was expressed in all urachal adenocarcinomas, 96% of colonic adenocarcinomas, and only 29% of the bladder neoplasms. The results of CEA expression in urachal and colonic carcinomas may represent the fact that the embryologic origin of the urachus and colon is the same [1]. Cytokeratin 7 expression was expressed in 82% of adenocarcinomas of the bladder, and focally in 40% of urachal and 38% of colonic adenocarcinomas. Cytokeratin 20 was expressed in more than 80% of all kinds of adenocarcinomas; therefore, cytokeratin 20 expression was not organ specific [2]. Suh et al. revealed that lack of CDX24 and villin signals strongly pointed to a primary bladder adenocarcinoma and AMACR is typically negative or weakly positive in urothelial carcinoma [3]. In this case, the result of immunohistochemistry of the tumor was not conclusive in demonstrating urachal adenocarcinoma. However, there was no clinical evidence of secondary involvement of adenocarcinoma from the bladder or colorectum.
AMACR is globally used as a reliable marker for prostate adenocarcinoma, but expression has also been demonstrated in various other neoplasms [4-6]. No AMACR expression in a urachal tumor has been reported. In our case, the tumor cells expressed AMACR, but were PSA negative by immunohistochemistry. Moreover, there was no clinical evidence of prostate adenocarcinoma. The expression of AMACR in this case may be aberrant, but could be a specific tumor marker for urachal adenocarcinoma. Thus, as far as we aware, this is the first report of urachal adenocarcinoma originating from the abdominal wall with expression of AMACR. Further examinations would be needed to clarify if AMACR expression is specific for urachal adenocarcinoma.
Figures and Tables
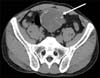 | FIG. 1Computed tomography through the lower abdomen showing a midline-lying, well circumscribed multilocular calcified cystic mass (arrow). |
 | FIG. 2Photomicrograph of the wall of the multilocular cystic mass shows mucin lakes by immunohistochemistry (H&E, ×100). |
Notes
References
1. Sheldon CA, Clayman RV, Gonzalez R, Williams RD, Fraley EE. Malignant urachal lesions. J Urol. 1984. 131:1–8.
2. Torenbeek R, Lagendijk JH, Van Diest PJ, Bril H, van de Molengraft FJ, Meijer CJ. Value of a panel of antibodies to identify the primary origin of adenocarcinomas presenting as bladder carcinoma. Histopathology. 1998. 32:20–27.
3. Suh N, Yang XJ, Tretiakova MS, Humphrey PA, Wang HL. Value of CDX2, villin, and alpha-methylacyl coenzyme A racemase immunostains in the distinction between primary adenocarcinoma of the bladder and secondary colorectal adenocarcinoma. Mod Pathol. 2005. 18:1217–1222.
4. Leav I, McNeal JE, Ho SM, Jiang Z. Alpha-methylacyl-CoA racemase (P504S) expression in evolving carcinomas within benign prostatic hyperplasia and in cancers of the transition zone. Hum Pathol. 2003. 34:228–233.
5. Xu J, Stolk JA, Zhang X, Silva SJ, Houghton RL, Matsumura M, et al. Identification of differentially expressed genes in human prostate cancer using subtraction and microarray. Cancer Res. 2000. 60:1677–1682.
6. Daugherty SE, Platz EA, Shugart YY, Fallin MD, Isaacs WB, Chatterjee N, et al. Variants in the alpha-Methylacyl-CoA racemase gene and the association with advanced distal colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2007. 16:1536–1542.
7. Yu JS, Kim KW, Lee HJ, Lee YJ, Yoon CS, Kim MJ. Urachal remnant diseases: spectrum of CT and US findings. Radiographics. 2001. 21:451–461.
8. Dandekar NP, Dalal AV, Tongaonkar HB, Kamat MR. Adenocarcinoma of bladder. Eur J Surg Oncol. 1997. 23:157–160.
9. Eble JN, Hull MT, Rowland RG, Hostetter M. Villous adenoma of the urachus with mucusuria: a light and electron microscopic study. J Urol. 1986. 135:1240–1244.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download