Abstract
Purpose
The aim of this study was to investigate the expression pattern of calcium-binding proteins S100A2 and S100A4. We also sought to determine the prognostic value of these markers for patients with prostate adenocarcinoma.
Materials and Methods
Immunohistochemical staining was performed to detect S100A2 and S100A4 expression in 26 tissue samples obtained during transurethral resection from patients with benign prostatic hyperplasia (BPH) and in 67 tissue samples obtained during prostate biopsy and radical prostatectomy from patients with prostate carcinoma. The immunoreactivity of these proteins was stratified on a scale of 0 to 3 and was correlated with the pathologic features of prostate adenocarcinoma.
Results
High expression of S100A2 was observed in the tissue of patients with BPH, whereas low or no expression was observed in prostate cancer (CaP) cells. The protein level of S100A4 was significantly higher in CaP than in BPH cells. The higher level of S100A4 observed in CaP tissue correlated with increasing tumor grade.
Prostate cancer (CaP) has the highest incidence among cancers in the US and is the second leading cause of cancer-associated death in men [1]. In Korea, its incidence is still increasing, compared to that of other cancers and it ranked fifth in incidence in 2005 [2]. Despite recent improvements in diagnosis and therapeutic techniques, the survival rate of CaP patients is poor because of the recurrence of the disease [1,3]. The lack of effective therapies for advanced CaP is related to a large extent to poor understanding of the molecular mechanisms underlying the progression of this disease (invasion and metastasis) [4]. Identification of predictive markers for CaP, especially those that are indicative of the invasiveness of the disease, is important for improving clinical management, outcomes, and survival of these patients. The S100 calcium-binding proteins have recently attracted considerable interest because of their differential expression in neoplastic and normal tissues, and their involvement in metastatic processes [5,6]. S100 proteins are involved in a variety of intracellular and extracellular functions including cell growth, cell-to-cell communication, energy metabolism, and intracellular signal transduction [6,7].
S100A2 is tumor-suppressor gene that is typically down-regulated in cells that have acquired a tumorigenic phenotype, which suggests that S100A2 has an important role in inhibiting cancer progression [8]. Differential expression of S100A2 has been reported in breast cancer, melanoma, and other types of cancer [9-14].
S100A4, another member of the S100 protein family, is associated with the invasion and metastasis of malignant tumors [15]. S100A4 is frequently overexpressed in metastatic tumors, as well as in normal cells with uninhibited movement, such as macrophages, neutrophils, and T-lymphocytes [16]. It is also overexpressed in various cancer types such as those of the breast, ovary, and colon [6,17,18].
In this study, we aimed to determine the expression pattern of S100A2 and S100A4 and to assess the prognostic value of these markers in patients with prostate adenocarcinoma.
Immunohistochemical staining for S100A2 and S100A4 was performed on 26 tissue samples obtained during transurethral resection from patients with benign prostatic hyperplasia (BPH) and 67 tissue samples obtained during prostate biopsy (n=7) and radical prostatectomy (n=60) from patients with prostate carcinoma. We divided the CaP tissue samples (n=67) into 3 groups based on their Gleason grade: Gleason grade 6 or lower (low grade) (n=24, 35.8%), Gleason grade 7 (intermediate grade) (n=24, 35.8%), and Gleason grade 8 or higher (high grade) (n=19, 28.4%). The samples were also assigned to one of three groups based on clinical stage: localized CaP (n=36, 53.7%), locally advanced CaP (n=13, 19.4%), and metastatic CaP (n=18, 26.9%).
Formalin-fixed, paraffin-embedded, 5 µm-thick sections were dewaxed in xylene, rehydrated in graded solutions of alcohol, and placed in an endogenous peroxide-blocking solution for 15 min. Sections were placed in a citrate buffer (10% citrate buffer stock in distilled water, pH 6.0). Nonreactive staining was blocked by incubation with 1% horse serum in Tris-buffered saline (pH 6.0) for 3 min. The primary antibodies for S100A2 (R&D Systems, Inc., Minneapolis, MN, USA) and S100A4 (Thermo Scientific, Waltham, MA, USA) were incubated with the sections overnight. Antibody-binding was detected by use of a standard labeled streptavidin-biotin system (Life Science Division, Mukilteo, USA). Breast cancer tissue was used as an external positive control. For negative controls, the primary antibodies were omitted.
Positive staining in the cytoplasm of tumor cells was defined as reddish-brown staining in at least 10% of the tumor cells in a section. A semiquantitative scale of 0 to 3 was used to score the reactivity the samples. The immunoreactive score was determined by the percentage of positive cells and the staining intensity, ranging from no detectable signal (0) to a strong signal seen at lower-power magnification (3). Each image was scored as 0 (0%, negative), 1 (10-30%, weak), 2 (30-70%, moderate), or 3 (70-100%, strong) and classified as low expression (score 0 and 1) and high expression (score 2 and 3) (Fig. 1, 2).
We examined the difference in S100A2 and S100A4 expression between BPH and CaP samples among the groups, which were classified based on localization of CaP. Biochemical progression-free survival was determined based on levels of prostate-specific antigen (PSA) and studied by use of the Kaplan-Meier product limit analysis. Differences between the groups were examined by use of the log-rank test. Biochemical recurrence was defined as an increase of 0.2 ng/ml. Comparisons of means within groups were made using the one-way ANOVA test. Comparisons of immunostaining scores within groups were made using the Kruskall-Wallis test. Sample correlations were estimated using Spearman's rank correlation. Statistical significance was defined as <0.05. All analyses were performed using the Statistical Package for Social Sciences (SPSS), version 12.0 for Windows.
The age of the patient did not differ significantly between patients with BPH (68.28±8.05 years) and CaP (68.69±8.26 years) (p=0.082) (Table 1). We examined the expression of S100A2 and S100A4 in benign and cancer tissues. We observed that the expression of S100A4 was significantly higher than that of S100A2 in CaP tissues (p<0.05) (Table 2). In BPH tissues, the expression of S100A2 was higher than that of S100A4 (p<0.05) (Table 2).
The age and follow up periods of patients did not differ significantly among the groups of CaP patients (p>0.05). We observed that the PSA level of patients was significantly different among groups according to clinical stage (p=0.023), but not significantly different among groups according to pathologic grade (p=0.264) (Table 1).
A significant progressive increase in S100A4 expression and decrease in S100A2 expression was observed in cancer specimens according to clinical stage and the pathologic grade (Gleason score) (p<0.05) (Table 3, 4, Fig. 1, 2). The Spearman correlation between S100A2 and S100A4 was found to be - 0.55 (p<0.001) based on mean that the total of every specimen.
Of the 67 patients with CaP, 10 (14.9%) had evidence of biochemical relapse and 4 (5.9%) died from prostate cancer. The mean follow-up period in our study was 32.3 months. The length of biochemical relapse-free survival among the 18 patients with immunoreactive scores of 2 or 3 (Group 2) for S100A2 was statistically longer than among the 49 patients with immunoreactive scores of 0 or 1 (Group 1) (log-rank, p=0.035) (Fig. 3). The length of biochemical relapse-free survival among the 26 patients with an immunoreactive score of 0 or 1 (Group 3) for S100A4 was statistically longer than among the 41 patients with an immunoreactive score of 2 or 3 (Group 4) for S100A4 (log-rank, p=0.021) (Fig. 4).
The S100 proteins are small acidic proteins (10-12 kDa) that are found exclusively in vertebrates [19]. With at least 25 members of this protein family identified to date in humans, the S100 proteins constitute the largest subfamily of EF-hand proteins. Of these, 21 family members (S100A1 - S100A18, trichohyalin, filaggrin and repetin) have genes clustered at chromosome locus 1q21; other S100 proteins are found at chromosome loci 4p16 (S100P), 5q14 (S100Z), 21q22 (S100B), and Xp22 (S100G). First identified by Moore in 1965, the S100 proteins exhibit 25-65% identity at the amino acid level and contain 2 EF-hand motifs flanked by conserved hydrophobic residues separated by a linker region [19,20]. The sequences of the linker region and the C-terminal extension are the most variable parts of the sequence among the S100 proteins. The S100 proteins are hypothesized to participate in signal trans duction pathways that regulate cell cycle progression and differentiation; however, the precise functions of these proteins are unknown.
The S100A2 gene encodes a protein of 99 amino acids. The gene is located on chromosome 1q21, in a region that is frequently rearranged in a number of human cancers [5,8]. Expression of S100A2 is regulated during the cell cycle, with its levels increasing as cells enter the S phase, and induced by growth factors in the early G1 phase of the normal cell cycle [8]. Because S100A2 is believed to be regulated by the tumor-suppressor p53, induction of p53 activity by cell cycle arrest caused by DNA damage results in increased S100A2 transcription [21,22]. The loss of S100A2 expression is associated with the development and progression of some human cancers [9-14]. Recently, S100A2 was proposed to be a class II tumor suppressor gene because of its loss of expression in a large number of tumors. This loss of expression is believed to influence the regulation of genes that are important for normal cell growth and differentiation [12,23]. In our study, we observed decreased S100A2 expression in CaP. It is important to emphasize that we observed decreased S100A2 expression only in CaP and not in BPH (nonmalignant).
Another member of the S100 family of proteins is S100A4, which influences cell cycle progression and cell motility, and modulates intracellular adhesion and invasiveness [15,16]. Expression of the S100A4 gene has been linked to invasion and metastasis of cancer cells and is upregulated in a number of human cancers [16,24]. Amplification or overexpression of S100A4 in patients with breast cancer reflects increased metastatic potential of the cancer, which is prognostically significant and closely correlated with death [25]. S100A4 has been reported to be expressed in 44% of foci of carcinoma within colon adenoma specimens and 94% of those of colon carcinoma specimens [26]. In our study, we observed high expression of S100A4 in invasive CaP. We also observed that decreased expression of S100A4 gene reduces the growth and proliferative potential of CaP. These results indicate that the S100A4 protein may be associated with proliferation, invasion, and metastasis during the progression of CaP.
During about 5 years of follow-up, 10 of 67 (14.9%) patients had a biochemical relapse based on PSA levels. We found that the length of biochemical relapse-free survival was longer in those patients with higher expression of S100A2 and shorter for those with higher expression of S100A4. This finding indicates that expression of S100A2 and S100A4 is significantly associated with prognosis.
Our study was limited by its retrospective design, the relatively small number of patients, and the short length of follow-up. This makes it difficult to draw definitive conclusions. Because a single pathologist assessed the immunohistochemical staining of samples in a blinded fashion, interobserver variance was not tested in this study.
We found an inverse relationship between expression of S100A2 and S100A4 protein in CaP and BPH. Reduced expression of S100A2 and increased expression of S100A4 in CaP was associated with clinical advancement and biologic aggressiveness of tumors. Hence, the simultaneous analysis of S100A2 and S100A4 expression in prostate tissues may be a useful prognostic marker for CaP.
Figures and Tables
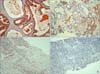 | FIG. 1Grading system used for in the expression of S100A2 (H&E, ×100). The degree of expression was graded on 4-point scale of 0 to 3: 0 (negative stain), 1+(weak staining), 2+(moderate staining), and 3+(strong staining). (A) Strong cytoplasmic expression of S100A2 was observed in benign prostatic hyperplasia tissue. (B) Moderate cytoplasmic expression of S100A2 was observed in CaP tissue (Gleason score 6). (C) There was weak cytoplasmic expression of S100A2 in CaP tissue (Gleason score 7). (D) Negative cytoplasmic expression of S100A2 in the CaP tissue (Gleason score 8). CaP: prostate cancer. |
 | FIG. 2Immunostaining of S100A4 protein in representative samples from cancer tissue with different degrees of progression of prostate cancer (CaP) (H&E, ×100). (A) The cytoplasmic expression of S100A4 in benign prostatic hyperplasia tissue was negative. (B) Weak in CaP tissue (Gleason score 6). (C) Moderate in CaP tissue (Gleason score 7). (D) There was strong cytoplasmic expression of S100A4 in CaP tissue (Gleason score 8). CaP: prostate cancer. |
 | FIG. 3Kaplan-Meier product-limit analysis of the length of biochemical relapse-free survival among CaP patients with low expression (IS=0 or 1) and high expression (IS=2 or 3) of S100A2 protein. CaP: prostate cancer, IS: immunoreactive score. |
 | FIG. 4Kaplan-Meier product-limit analysis of the length of biochemical relapse-free survival among prostate cancer patients with low expression (IS=0 or 1) and high expression (IS=2 or 3) of S100A4 protein. CaP: prostate cancer, IS: immunoreactive score. |
Notes
References
1. Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006. 56:106–130.
2. Ministry for Health, Welfare and Family Affairs. Annual report of cancer incidence (2005) and survival (1993-2005) in Korea. 2008. Seoul: The Ministry.
3. Klein EA, Thompson IM. Update on chemoprevention of prostate cancer. Curr Opin Urol. 2004. 14:143–149.
4. Saleem M, Adhami VM, Zhong W, Longley BJ, Lin CY, Dickson RB, et al. A novel biomarker for staging human prostate adenocarcinoma: overexpression of matriptase with concomitant loss of its inhibitor, hepatocyte growth factor activator inhibitor-1. Cancer Epidemiol Biomarkers Prev. 2006. 15:217–227.
5. Ilg EC, Schäfer BW, Heizmann CW. Expression pattern of S100 calcium-binding proteins in human tumors. Int J Cancer. 1996. 68:325–332.
6. Heizmann CW. The multifunctional S100 protein family. Methods Mol Biol. 2002. 172:69–80.
7. Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001. 33:637–668.
8. Lee SW, Tomasetto C, Swisshelm K, Keyomarsi K, Sager R. Down-regulation of a member of the S100 gene family in mammary carcinoma cells and reexpression by azadeoxycytidine treatment. Proc Natl Acad Sci U S A. 1992. 89:2504–2508.
9. Liu D, Rudland PS, Sibson DR, Platt-Higgins A, Barraclough R. Expression of calcium-binding protein S100A2 in breast lesions. Br J Cancer. 2000. 83:1473–1479.
10. Maelandsmo GM, Florenes VA, Mellingsaeter T, Hovig E, Kerbel RS, Fodstad O. Differential expression patterns of S100A2, S100A4 and S100A6 during progression of human malignant melanoma. Int J Cancer. 1997. 74:464–469.
11. Nagy N, Hoyaux D, Gielen I, Schafer BW, Pochet R, Heizmann CW, et al. The Ca2+-binding S100A2 protein is differentially expressed in epithelial tissue of glandular or squamous origin. Histol Histopathol. 2002. 17:123–130.
12. Feng G, Xu X, Youssef EM, Lotan R. Diminished expression of S100A2, a putative tumor suppressor, at early stage of human lung carcinogenesis. Cancer Res. 2001. 61:7999–8004.
13. Bronckart Y, Decaestecker C, Nagy N, Harper L, Schafer BW, Salmon I, et al. Development and progression of malignancy in human colon tissues are correlated with expression of specific Ca(2+)-binding S100 proteins. Histol Histopathol. 2001. 16:707–712.
14. Lauriola L, Michetti F, Maggiano N, Galli J, Cadoni G, Schafer BW, et al. Prognostic significance of the Ca(2+) binding protein S100A2 in laryngeal squamous-cell carcinoma. Int J Cancer. 2000. 89:345–349.
15. Sherbet GV, Lakshmi MS. S100A4 (MTS1) calcium binding protein in cancer growth, invasion and metastasis. Anticancer Res. 1998. 18:2415–2421.
16. Grigorian MS, Tulchinsky EM, Zain S, Ebralidze AK, Kramerov DA, Kriajevska MV, et al. The mts1 gene and control of tumor metastasis. Gene. 1993. 135:229–238.
17. Taylor S, Herrington S, Prime W, Rudland PS, Barraclough R. S100A4 (p9Ka) protein in colon carcinoma and liver metastases: association with carcinoma cells and T-lymphocytes. Br J Cancer. 2002. 86:409–416.
18. Garrett SC, Varney KM, Weber DJ, Bresnick AR. S100A4, a mediator of metastasis. J Biol Chem. 2006. 281:677–680.
19. Schafer BW, Heizmann CW. The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci. 1996. 21:134–140.
20. Moore BW. A soluble protein characteristic of the nervous system. Biochem Biophys Res Commun. 1965. 19:739–744.
21. Sun Y. Identification and characterization of genes responsive to apoptosis: application of DNA chip technology and mRNA differential display. Histol Histopathol. 2000. 15:1271–1284.
22. Tan M, Heizmann CW, Guan K, Schafer BW, Sun Y. Transcriptional activation of the human S100A2 promoter by wild-type p53. FEBS Lett. 1999. 445:265–268.
23. Nagy N, Brenner C, Markadieu N, Chaboteaux C, Camby I, Schafer BW, et al. S100A2, a putative tumor suppressor gene, regulates in vitro squamous cell carcinoma migration. Lab Invest. 2001. 81:599–612.
24. Barraclough R. Calcium-binding protein S100A4 in health and disease. Biochim Biophys Acta. 1998. 1448:190–199.
25. Rudland PS, Platt-Higgins A, Renshaw C, West CR, Winstanley JH, Robertson L, et al. Prognostic significance of the metastasis-inducing protein S100A4 (p9Ka) in human breast cancer. Cancer Res. 2000. 60:1595–1603.
26. Takenaga K, Nakanishi H, Wada K, Suzuki M, Matsuzaki O, Matsuura A, et al. Increased expression of S100A4, a metastasis-associated gene, in human colorectal adenocarcinomas. Clin Cancer Res. 1997. 3:2309–2316.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download