Abstract
Purpose
The incidence of adenocarcinoma on a subsequent biopsy following a diagnosis of atypical small acinar proliferation (ASAP) ranges from 34% to 60%. We reexamined radical prostatectomy (RP) specimens of patients diagnosed as having synchronous ASAP with prostate cancer (PCa) to evaluate pathological entities and the clinical significance of ASAP.
Materials and Methods
From January 2007 to December 2008, a total of 118 patients who had been diagnosed with adenocarcinoma on prostate needle biopsy underwent RP. Forty-six of the 118 patients (39%) were diagnosed as having synchronous ASAP with PCa on the prostate needle biopsy. Using whole-mount sections and prostate mapping, we evaluated the RP specimens that were close sections to the ASAP on prostate needle biopsy. All tissues were examined by immunohistochemistry with high molecular weight cytokeratin (34βE12), p63, and AMACR/P504S added to initial H&E stains by one pathologist.
Results
Thirty-six of the 46 patients (78%) were diagnosed as having adenocarcinoma at sites of ASAP on the initial prostate needle biopsies. The Gleason score was 5 to 6 in 22 patients (61%), 7 in 3 (8%), and unknown due to multifocal and microfocal lesions in 11 (31%). The tumor volume of 14 of the 36 patients (39%) was 0.5 cc or less and was unknown due to multifocal and microfocal lesions in 8 (22%).
The term 'atypical small acinar proliferation (ASAP)' was introduced by Bostwick et al to indicate suspicious glands with insufficient cytological or architectural atypia for the diagnosis of a definitive adenocarcinoma in prostate biopsies [1]. The incidence of ASAP in prostate biopsies was 0.4% to 23.4% in 12 consecutive studies [2]. The clinical significance of ASAP is its predictive value for prostate cancer (PCa) in a repeat biopsy, which ranged from 34% to 60% in similar studies [3,4]. On this account, current recommendations for patients with ASAP in their initial biopsy are subsequent biopsy within 3 to 6 months irrespective of follow-up prostate-specific antigen (PSA) values [5].
Immunohistochemical stains with benign prostate basal cell markers such as high molecular weight cytokeratin (34βE12) or p63 and markers of cancer such as alpha-methylacyl CoA racemase (AMACR/P504S) have been used for the differential diagnosis of ASAP and adenocarcinoma, although neither stain confirms a definitive diagnosis for PCa [6-8]. In addition, contemporary prostate biopsy protocols have increased the number of cores from sextant biopsy to extended biopsy [9]. A recent report suggested that these advances have reduced the predictive value of ASAP for subsequent cancer to 37% [10]. By contrast, a report of immediate prostatectomies after isolated ASAP was diagnosed described a 100% predictive value of ASAP [11]. There are many differences in the pathological entities and clinical significance of ASAP between studies.
We reviewed radical prostatectomy (RP) specimens in patients with synchronous ASAP with PCa on prostate needle biopsy to evaluate the pathological entities and, by extension, the clinical significance of ASAP.
From January 2007 to December 2008, we conducted systematic transrectal ultrasound (TRUS)-guided prostate needle biopsies at our institution because of increased serum PSA values or abnormality on a digital rectal examination (DRE). Prostate biopsies were acquired under TRUS (IU-22; PHILIPS, USA) guidance by using a biopsy gun (ACECUT; TSK Laboratory, Japan) with an 18 gauge needle by one radiologist. We performed 6-core biopsies [12] in patients with a TRUS volume of less than 30 cc and 12-core biopsies [13] in patients with a TRUS volume of 30 cc or more. All specimens were stained with H&E to evaluate features considered specific for carcinoma. In addition, immunohistochemical stains such as 34βE12, p63, and AMACR/P504S were used for the differential diagnosis of ASAP and carcinoma. All tissues were evaluated by one pathologist.
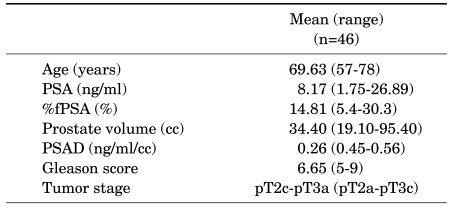
A total of 118 patients underwent RP because they were diagnosed with PCa on the prostate needle biopsy at our institution. Forty-six of the 118 patients (39%) were diagnosed as having synchronous ASAP with PCa on the prostate needle biopsy. In those 46 patients, the mean age was 69.63 years (range, 57-78 years), the mean PSA level before the prostate biopsy was 8.17 ng/ml (range, 1.75-26.89 ng/ml), and the mean prostate volume was 34.40 cc (range, 19.10-95.40 cc). The RP pathology results (Table 1) showed that the patients had a mean Gleason score of 6.65 (range, 5-9) and a mean pathological stage of pT2c-pT3a (range, pT2a-pT3c).
All specimens acquired from RP were mapped by using complete sampling with whole-mount sections [14]. Using prostate mapping, we retrospectively evaluated the sites in the RP specimens that had been diagnosed as ASAP on prostate needle biopsy. All RP specimens were examined with the same H&E stain and immunohistochemical stains by one pathologist who had previously evaluated the prostate needle biopsies. For measurement of tumor volume, visual inspection and grid morphometric analysis were done (tumor volume=1.25 × mean percentage carcinoma × number of block).
Student's t-test was used to assess the clinical values. Statistical analysis was performed by using statistical software (SPSS 10.1; SPSS Inc., USA), and a p-value of less than 0.05 was considered to indicate a statistically significant difference.
Thirty-six of the 46 patients (78%) who underwent RP were shown to have adenocarcinoma at the sites diagnosed as ASAP on the initial prostate needle biopsy. The characteristics of these 36 patients and those of the other 10 patients who were not diagnosed are shown in Table 2. Only pathological stage was statistically significantly different between the groups (p<0.005).
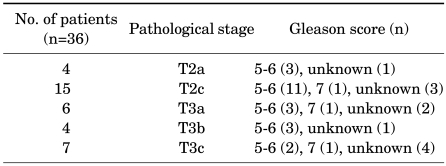
Of the 36 patients, pathological stage was T2a in 4 patients, T2c in 15, T3a in 6, T3b in 4, and T3c in 7. Four of the 36 patients (11%) had lesions located at one lobe and 19 of them (53%) were organ-confined disease.
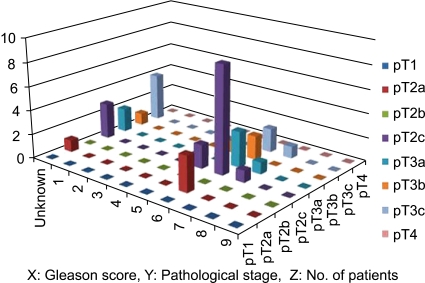
The Gleason score was 5 to 6 in 22 patients (61%), 7 in 3 (8%), and unknown in 11 (31%). The patients with an unknown Gleason score had cancerous features but a Gleason score could not be assigned because of multifocal and very small lesions. In particular, of the 25 patients who were assigned a Gleason score, 22 (88%) were diagnosed with a Gleason score of 6 or less. Table 3 and Fig. 1 summarize the pathological stages and Gleason scores in the 36 patients.
Of the 36 patients, the mean tumor volume of 28 of the patients in whom we could assess tumor volume was 2.5 cc, and 14 of the 28 patients (50%) had a tumor volume of 0.5 cc or less. Eight of the 36 patients could not be evaluated because of multifocal lesions.
The 10 patients not diagnosed as having PCa (at the site of the ASAP on the initial needle biopsy) had pT2a (9 patients), pT3a (1 patient), a Gleason score of 6 (7 patients), and a Gleason score of 7 (3 patients) in the RP pathology results.
PCa is diagnosed by prostate needle biopsy when patients have an elevated serum PSA level, an abnormality on the digital rectal examination, or a low echoic lesion on TRUS. Park et al reported that among 1,045 men who underwent TRUS-guided systematic needle biopsy, 104 underwent repeat prostate biopsies after a previous negative biopsy, and PCa was detected in 22 (21.2%) of the 104 patients [15]. A false-negative result on the initial biopsy or a delayed diagnosis can result because of the difficulty of diagnosing cancer from a prostate needle biopsy specimen. This difficulty is due to the fact that quantitative and qualitative changes are necessary for a pathological diagnosis of adenocarcinoma, the amount of prostatic tissue acquired by needle-core biopsy is limited, and a certain degree of subjectivity is involved in the pathological diagnosis [11]. Therefore, ASAP has commonly been used to refer to an atypical focus suspicious but not diagnostic of malignancy [3]. The terminology of ASAP encompasses various lesions including benign lesions, small acinar proliferations mimicking PCa, and atypical glandular proliferations suspicious for carcinoma, which cannot be accurately diagnosed for a various reasons such as insufficient amount of specimen or biopsy-induced mechanical distortion [16]. Despite the popular recognition of ASAP as a pathological diagnosis, criticism does exist. First, ASAP legitimizes pathological uncertainty by reporting it like a diagnosis. Second, ASAP may have differences of opinion between independent pathologists. Concordance rates of 84% and 78% between independent pathologists were described in previous studies [17]. Third, several large studies reported a subsequent biopsy rate of <70% after a diagnosis of ASAP on the first prostate biopsy [18-20]. Finally, delayed diagnosis can result because the term ASAP does not adequately convey the seriousness of the biopsy result to either the urologist or the patient [17]. Even though ASAP might be incomplete in terms of pathological diagnosis, its predictive values for PCa on repeat biopsy ranged from 34% to 60% in previous studies [3,4]. We identified PCa postoperatively in 78% of ASAP identified in the initial prostate needle biopsy.
Brausi et al reported that 25 of 71 patients with isolated ASAP underwent immediate RP and 23 of them underwent subsequent biopsy. All 25 patients (100%) who underwent RP had a final pathological diagnosis of adenocarcinoma and 9 of the 23 patients (39.1%) who underwent follow-up biopsies were diagnosed as having adenocarcinoma in 6 to 12 months after the initial diagnosis of ASAP. Those authors suggested RP as one of the treatment choices in young patients with isolated ASAP on prostate needle biopsy. In their study, 9 of the 25 patients (36%) had a tumor localized to a single lobe (pT2a) with a Gleason score of 2 to 6, and the cancer volume was less than 0.5 cc in 3 of these 9 patients. Even though 4 (16%) patients had cancers with a Gleason score of 7 or more, the others had relatively low-grade cancer, which might indicate clinically insignificant PCa [11].
In another study, Flury et al reviewed whole-tissue sections of prostates removed at 65 cystoprostatectomies [21]. They reported that 2 of 10 ASAP foci were diagnosed as adenocarcinoma with additional sectioning of the corresponding tissue block. In addition, 6 of 10 ASAP foci had immunohistochemistry supporting a cancer diagnosis, but not enough material for such a diagnosis. Two foci that were diagnosed as PCa had a total volume of much less than 0.5 cc and a Gleason score of 6, respectively. Those authors proposed that ASAP represented clinically insignificant PCa or marginally sampled PCa and that aggressive diagnostic approaches to seek PCa were necessary whenever ASAP is seen on prostate needle biopsy [21]. If a carcinoma is marginally or imperfectly sampled, the microscopic focus might be very small and might contain only a small number of acini [3]. This is certainly a contributory factor to having much difficulty in evaluating specimens.
Previous studies described the adenocarcinomas found on follow-up biopsy to be of various grades, 18% to 71% had Gleason scores of 5 or 6, and 29% to 82% were PCa with Gleason scores of 7 or more [17,22,23]. Ultimately, ASAP represented 'a marginally sampled, tangentially sectioned or out-pouching adenocarcinoma' [24].
The Epstein criteria are currently one of the most widely used definitions for clinically insignificant PCa. These are composed of PSA density of 0.15 ng/ml2 or less, Gleason score of 6 or less, fewer than three biopsies with PCa, and up to 50% of cancer involvement in any core. In another study, clinically insignificant cancer was defined as that with an RP tumor volume of less than 0.5 cc, a Gleason score of 6 or less, and organ-confined cancer [25]. van Oort et al reported that the biological behavior of small volume and insignificant PCa was significantly more favorable than that of a general group of patients undergoing RP [26]. For that reason, patients with such insignificant cancer may be appropriate candidates for active surveillance rather than initial radical therapies. But, the plain fact is that the Epstein criteria cannot predict clinically insignificant PCa accurately in some cases and may underestimate the true nature of PCa in 30.5% of Korean patients and 24% of European patients [27,28].
In our study, 61% of the patients diagnosed as having PCa had low-grade cancer with a Gleason score of 5 or 6 and 8% of them had intermediate-grade cancer with a Gleason score of 7. In 31% of our patients, the Gleason score could not be evaluated because of multifocal and very small lesions. Also, 39% of the patients diagnosed as having PCa had a tumor volume smaller than 0.5 cc. Together with the aforementioned studies, our data strongly suggest that ASAP represents clinically insignificant PCa or marginally sampled PCa.
Mechanical distortion from the needle biopsy can result in crush artifact of a few atypical glands and obscure cytologic detail. Problems with fixation and processing can prevent a definite diagnosis because of poor histological features [3]. We suggest that alteration of cytological features could result from needle-core biopsy, especially in small-volume foci and that these were some of the reasons we did not find significant features in 10 patients even though the RP specimens contained sufficient tissue.
Matsumoto et al found a 7.7% increase in cancer detection by use of an additional far lateral sextant biopsy in the peripheral zone, and the 12-core biopsy scheme yielded a 7.7% to 13.8% increase in cancer detection in a Japanese series [29]. Furthermore, a recent study by Ploussard et al reported that the 21-core extended biopsy improved the diagnostic yield of 39.1% for ASAP compared with 12-core biopsy and cancers were found at the same sample site (71%) and side (100%) by using subsequent 21-core biopsy of patients with an initial diagnosis of ASAP [30]. In another study, it was reported that sampling only the side or sextant site with ASAP would have failed to detect cancer in 39% of patients with cancer detected exclusively in other sites [22]. Therefore, our data and the aforementioned studies suggest that aggressive extended sampling will do better to include additional biopsy of the site of ASAP and saturation biopsy.
Several limitations to our study exist. Because isolated ASAP is not a generally accepted indication of RP, we evaluated RP specimens with synchronous ASAP with adenocarcinoma and could not evaluate RP specimens with isolated ASAP. Therefore, the diagnosis rates of adenocarcinoma were different between a previous study [11] and ours (100% vs. 78%). But there was no difference between the characteristics of cancer, such as low grade and small volume caner. In addition, only a limited number of patients (46) underwent RP due to synchronous ASAP with PCa. Also, because we did not routinely perform RP in high-risk patients, which was defined as clinical stage T3b or higher disease or PSA greater than 20 ng/ml or a Gleason score of 8 or greater, advanced PCa was excluded from the beginning of our study. The context results in no significant differences in Gleason pattern between PCa on reexamining whole tissue sections from the site of ASAP and PCa on needle prostate biopsy.
Although the pathological entities and clinical significance of ASAP on prostate biopsies have been investigated comprehensively, a few studies have reported associations between ASAP on prostate needle biopsy and adenocarcinoma in whole tissue sections of surgery. The results of our study show that ASAP is most often marginally sampled cancer or clinically insignificant PCa. Therefore, we suggest that aggressive diagnostic approaches like additional biopsy of the site of the ASAP or more extended biopsy be implemented as soon as possible if ASAP is diagnosed by prostate needle biopsy.
References
1. Bostwick DG, Srigley J, Grignon D, Maksem J, Humphrey P, van der Kwast TH, et al. Atypical adenomatous hyperplasia of the prostate: morphologic criteria for its distinction from well-differentiated carcinoma. Hum Pathol. 1993; 24:819–832. PMID: 8375853.

2. Epstein JI, Potter SR. The pathological interpretation and sig nificance of prostate needle biopsy findings: implications and current controversies. J Urol. 2001; 166:402–410. PMID: 11458037.
3. Montironi R, Scattoni V, Mazzucchelli R, Lopez-Beltran A, Bostwick DG, Montorsi F. Atypical foci suspicious but not diagnostic of malignancy in prostate needle biopsies (also referred to as "atypical small acinar proliferation suspicious for but not diagnostic of malignancy"). Eur Urol. 2006; 50:666–674. PMID: 16930809.
4. Oh JW, Kim YB, Yang SO, Lee JK, Kim YJ, Jung TY, et al. Prostate cancer detection rate of rebiopsy in patients with an initial diagnosis of atypical small acinar proliferation of the prostate. Korean J Urol. 2009; 50:237–240.

5. Ramey JR, Halpern EJ, Gomella LG. Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Further commentary: ultrasonography and biopsy of the prostate. Campbell-Walsh urology. 2007. 9th ed. Philadelphia: Saunders;p. 2883–2895.
6. Yun JH, Cho HD, Kim DS, Lee CH. The usefulness of P504S/34βE12 immunostaining for the detection of prostate cancer. Korean J Urol. 2007; 48:677–683.

7. Jiang Z, Li C, Fischer A, Dresser K, Woda BA. Using an AMACR (P504S)/34betaE12/p63 cocktail for the detection of small focal prostate carcinoma in needle biopsy specimens. Am J Clin Pathol. 2005; 123:231–236. PMID: 15842047.
8. Zhou M, Aydin H, Kanane H, Epstein JI. How often does alpha-methylacyl-CoA-racemase contribute to resolving an atypical diagnosis on prostate needle biopsy beyond that provided by basal cell markers? Am J Surg Pathol. 2004; 28:239–243. PMID: 15043314.

9. Iczkowski KA. Current prostate biopsy interpretation: criteria for cancer, atypical small acinar proliferation, high-grade prostatic intraepithelial neoplasia, and use of immunostains. Arch Pathol Lab Med. 2006; 130:835–843. PMID: 16740037.

10. Schlesinger C, Bostwick DG, Iczkowski KA. High-grade prostatic intraepithelial neoplasia and atypical small acinar proliferation: predictive value for cancer in current practice. Am J Surg Pathol. 2005; 29:1201–1207. PMID: 16096410.
11. Brausi M, Castagnetti G, Dotti A, De Luca G, Olmi R, Cesinaro AM. Immediate radical prostatectomy in patients with atypical small acinar proliferation. Over treatment? J Urol. 2004; 172:906–908. PMID: 15310995.

12. Hodge KK, McNeal JE, Terris MK, Stamey TA. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol. 1989; 142:71–74. PMID: 2659827.

13. Elabbady AA, Khedr MM. Extended 12-core prostate biopsy increases both the detection of prostate cancer and the accuracy of Gleason score. Eur Urol. 2006; 49:49–53. PMID: 16314035.

14. Bostwick DG, Montironi R. Evaluating radical prostatectomy specimens: therapeutic and prognostic importance. Virchows Arch. 1997; 430:1–16. PMID: 9037309.

15. Park SJ, Miyake H, Hara I, Eto H. Predictors of prostate cancer on repeat transrectal ultrasound-guided systematic prostate biopsy. Int J Urol. 2003; 10:68–71. PMID: 12588600.

16. Samaratunga H, Gardiner RA, Yaxley J, Brown I. Atypical prostatic glandular proliferations on needle biopsy: diagnostic implications, use of immunohistochemistry, and clinical significance. Anal Quant Cytol Histol. 2006; 28:104–110. PMID: 16637513.
17. Mancuso PA, Chabert C, Chin P, Kovac P, Skyring T, Watt WH, et al. Prostate cancer detection in men with an initial diagnosis of atypical small acinar proliferation. BJU Int. 2007; 99:49–52. PMID: 17227491.

18. Fadare O, Wang S, Mariappan MR. Practice patterns of clinicians following isolated diagnoses of atypical small acinar proliferation on prostate biopsy specimens. Arch Pathol Lab Med. 2004; 128:557–560. PMID: 15086303.

19. Iczkowski KA, Chen HM, Yang XJ, Beach RA. Prostate cancer diagnosed after initial biopsy with atypical small acinar proliferation suspicious for malignancy is similar to cancer found on initial biopsy. Urology. 2002; 60:851–854. PMID: 12429314.

20. Chan TY, Epstein JI. Follow-up of atypical prostate needle biopsies suspicious for cancer. Urology. 1999; 53:351–355. PMID: 9933053.

21. Flury SC, Galgano MT, Mills SE, Smolkin ME, Theodorescu D. Atypical small acinar proliferation: biopsy artefact or distinct pathological entity? BJU Int. 2007; 99:780–785. PMID: 17378841.

22. Iczkowski KA, Bassler TJ, Schwob VS, Bassler IC, Kunnel BS, Orozco RE, et al. Diagnosis of "suspicious for malignancy" in prostate biopsies: predictive value for cancer. Urology. 1998; 51:749–757. PMID: 9610588.

23. Park S, Shinohara K, Grossfeld GD, Carroll PR. Prostate cancer detection in men with prior high grade prostatic intraepithelial neoplasia or atypical prostate biopsy. J Urol. 2001; 165:1409–1414. PMID: 11342887.

24. Vis AN, Van Der Kwast TH. Prostatic intraepithelial neoplasia and putative precursor lesions of prostate cancer: a clinical perspective. BJU Int. 2001; 88:147–157. PMID: 11446873.

25. Allan RW, Sanderson H, Epstein JI. Correlation of minute (0.5 MM or less) focus of prostate adenocarcinoma on needle biopsy with radical prostatectomy specimen: role of prostate specific antigen density. J Urol. 2003; 170:370–372. PMID: 12853777.

26. van Oort IM, Kok DE, Kiemeney LA, Hulsbergen-van de Kaa CA, Witjes JA. A single institution experience with biochemical recurrence after radical prostatectomy for tumors that on pathology are of small volume or "insignificant". Urol Oncol. 2009; 27:509–513. PMID: 18625570.

27. Lee SE, Kim DS, Lee WK, Park HZ, Lee CJ, Doo SH, et al. Application of the Epstein criteria for prediction of clinically insignificant prostate cancer in Korean men. BJU Int. 2009; Epub ahead of print.

28. Jeldres C, Suardi N, Walz J, Hutterer GC, Ahyai S, Lattouf JB, et al. Validation of the contemporary epstein criteria for insignificant prostate cancer in European men. Eur Urol. 2008; 54:1306–1313. PMID: 18083294.

29. Matsumoto K, Satoh T, Egawa S, Shimura S, Kuwao S, Baba S. Efficacy and morbidity of transrectal ultrasound-guided 12-core biopsy for detection of prostate cancer in Japanese men. Int J Urol. 2005; 12:353–360. PMID: 15948721.

30. Ploussard G, Plennevaux G, Allory Y, Salomon L, Azoulay S, Vordos D, et al. High-grade prostatic intraepithelial neoplasia and atypical small acinar proliferation on initial 21-core extended biopsy scheme: incidence and implications for patient care and surveillance. World J Urol. 2009; 27:587–592. PMID: 19373471.

TABLE 2
Patient characteristics after radical prostatectomy
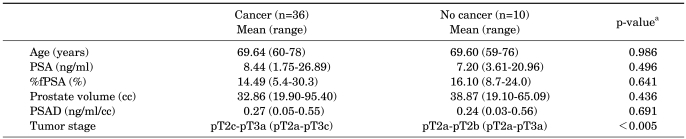
PSA: prostate-specific antigen, %fPSA: % free PSA, PSAD: PSA density, Cancer: patients were revealed as PCa at the sites diagnosed as ASAP on initial prostate needle biopsy, No cancer: patients were not revealed as PCa at the sites diagnosed as ASAP on initial prostate needle biopsy, a: Student's t-test




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download