Abstract
A 61-year-old man who had been diagnosed with prostate cancer 9 years ago and had been treated with pelvic irradiation and intermittent androgen deprivation therapy visited the emergency room because of back pain and weakness in both legs. Spine magnetic resonance imaging showed a lumbar epidural mass and spine metastasis. The whole-body workup revealed multiple metastases to the lymph nodes, bone, liver, and lung. The serum prostate-specific antigen was 0.02 ng/ml. He underwent laminectomy, posterior fixation, and epidural mass excision, and metastatic adenocarcinoma from the prostate was diagnosed. The patient underwent 1 cycle of docetaxel-based chemotherapy. More chemotherapy could not be done because of his general weakness. The patient died one month later of multiple organ failure.
The serum prostate-specific antigen (PSA) level has been used since the 1980s to monitor patients after treatment for prostate cancer. The kinetics of PSA after the treatment of prostate cancer make it a sensitive marker for the activity and status of this disease. Many studies have suggested that PSA may be the most valuable factor for predicting the activity and volume of prostate cancer [1].
Almost all patients with metastatic prostate cancer present with a high serum PSA level. However, progression of prostate cancer can sometimes occur despite a low serum PSA level. Those patients with metastatic prostate cancer and a low serum level of PSA account for less than 1% of all patients with metastatic prostate cancer [2]. There have been only a few reports about these patients. In these cases, androgen deprivation therapy (ADT) is relatively ineffective, and the prostate cancer progresses very quickly [3]. Therefore, the prognosis is generally poor compared with the usual metastatic prostate cancer. Here we report the case of a patient who had multiple sites of metastatic prostate cancer and a low serum level of PSA during intermittent ADT. We also review the related literature.
A 52-year-old male patient visited our institute for lower urinary tract symptoms on 18, September 2000. His initial PSA level was 21.6 ng/ml. A firm, nontender prostate with enlarged lobes was found on the digital rectal examination. Prostate biopsy showed the tumor to be an adenocarcinoma with a Gleason score of 8 (4+4) (Fig. 1A). Prostate magnetic resonance imaging (MRI) and a whole-body bone scan were subsequently performed and the patient was diagnosed as having a clinical stage T1cN0M0 prostate adenocarcinoma.
The patient and his family desired a nonsurgical treatment, and we recommended radiation therapy with ADT. He underwent 64.8 Gy of whole-pelvis irradiation for prostate cancer. He underwent adjuvant ADT for the first 3 years and then he underwent intermittent ADT for the next 5 years.
His serum PSA level was regularly checked. His highest PSA level was 3.05 ng/ml and the nadir PSA was 0.02 ng/ml. The PSA level dropped to 0.02 ng/ml at 7 years after radiation therapy and this level persisted (Fig. 2). We performed abdomen and pelvis computed tomography (CT) and whole-body bone scanning 4 and 7 years ago and found no evidence of recurrence.
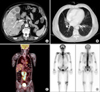
Nine years after the radiotherapy, he presented with lower back pain and weakness in both legs. Spine MRI revealed a lumbar epidural mass and spine metastasis with a compression fracture. The whole-body bone scan, positron emission tomography, and abdomen, pelvis, and chest CT revealed multiple metastases to the lymph nodes, bone, liver, and lung (Fig. 3). The patient's serum PSA level was only 0.02 ng/ml. He underwent laminectomy, posterior fixation, and epidural mass excision; he was diagnosed with metastatic adenocarcinoma and he had a Gleason score of 10 (5+5) (Fig. 1B). The malignant cells were negative for synaptophysin, chromogranin A, and CD56. Therefore, immunohistochemical studies were not consistent with a diagnosis of neuroendocrine or small cell prostate cancer. He was examined for other serum tumor markers. The values of carcinoembryonic antigen (CEA), α-fetoprotein (αFP), and β-human chorionic gonadotropin (β-HCG) were all within the normal ranges. However, CA 19-9 was 73.47 U/ml and CA 15-3 was 25.98 U/ml, and these values were slightly higher than the normal range. He was also examined via duodenoscopy and colonoscopy, which showed no evidence of malignancy.
He received 1 cycle of docetaxel-based chemotherapy. After chemotherapy, his general weakness was aggravated, so no more chemotherapy could be done. He died one month later of multiple organ failure.
ADT as a treatment for patients with prostate cancer was started 60 years ago. Monitoring the level of PSA has created a dramatic shift in the population of patients for whom androgen ablation is initiated. This has resulted in several changing concepts of treatment. Patients with recurrent prostate cancer after the failure of local therapy are now being newly diagnosed with recurrent disease on the basis of a rising PSA level.
The PSA level is the most valuable tool for monitoring disease status and the treatment response, especially in patients with advanced metastatic prostate cancer, as shown in many studies [1,4,5]. However, the progression of prostate cancer can sometimes occur in the presence of an undetectable or low serum PSA level. Yamamoto et al reported on 8 patients with clinically assessed M1 prostate cancer, and their serum PSA levels were less than 10 ng/ml. Most of them had poorly differentiated or undifferentiated tumors and they had a poor prognosis compared with the usual M1 prostate cancer. Yamamoto et al reported that ADT was ineffective in these cases and therefore that systemic chemotherapy and irradiation therapy should be recommended as the initial therapy [3].
Sella et al reported on 18 patients with clinically progressive androgen-independent prostate cancer and low PSA levels. Each patient demonstrated elevation of at least one tumor marker, such as CEA, CA 19-9, CA 15-3, and CA 125. Neuroendocrine immunoreactivity was detected in all specimens. They reported that progressive androgen-independent prostate cancer and a low serum PSA level was characterized by visceral metastasis, a high proportion of lytic bone disease, sensitivity to cisplatin-based chemotherapy, and the histological features of small cell or poorly differentiated prostate cancer [6]. Nishio et al also reported that serum CEA, CA 19-9, and CA 15-3 were elevated in patients with metastatic prostate cancer who had normal serum PSA levels [7].
Compared with the previous reports of metastatic prostate cancer patients with low PSA levels, our case presented with a high-grade Gleason score, a low PSA level of less than 0.1 ng/ml, visceral metastasis, and elevated levels of the tumor markers CA 19-9 and CA 15-3. However, there was no neuroendocrine component.
Given the biological heterogeneity of prostate cancer and its multifocality, it is possible that tumors with a higher Gleason grade have a greater chance for occurrence of a clonal shift in the original tumor that may express the features of other prostate cancers, including biological aggressiveness, and that such tumors may be associated with a lack of PSA production. The phenomenon of prostate cancer progression without concomitant PSA elevation may be explained by the proliferation of cell lines that either cannot produce PSA or are poorly differentiated prostate cancer cells that have lost their ability to express PSA. Magklara et al reported that both PSA and human kallikrein 2 are expressed more in noncancerous prostatic tissue than in cancerous prostatic tissue [8].
Only limited data are available to analyze the reason for recurrent prostate cancer with a low serum PSA. The limited reported experience suggests that the prognosis of patients with recurrence and without detectable serum PSA is variable. Oefelein et al reported that patients who had prostate cancer progression with undetectable serum PSA levels after radical prostatectomy had rapid progression [9].
In conclusion, prostate cancer may progress despite low serum PSA levels. Although this is an uncommon clinical scenario, patients with a high-grade Gleason score and undifferentiated tumors can progress to this event. A complete physical evaluation, including a digital rectal examination, imaging studies, and measurement of the serum PSA level, may be helpful in the follow-up of patients with these prostate cancers.
Figures and Tables
FIG. 1
(A) Histology of prostate adenocarcinoma of Gleason's grade 8 (4+4) 9 years previously. Irregular sizes of glands and fusioned glands are shown (H&E, ×400). (B) Histology of epidural metastatic adenocarcinoma of Gleason's grade 10 (5+5). No glandular differentiation and focal necrosis are shown (H&E, ×400).

FIG. 2
Clinical course of the patient. The serum prostate-specific antigen (PSA) level declined after irradiation and androgen deprivation therapy (ADT). The highest PSA was 3.05 ng/ml. Since 2007, the serum PSA level was 0.02 ng/ml.

FIG. 3
Multiple metastatic prostate cancer in the patient. (A) Abdomen and pelvic computed tomography (CT) showing multiple hepatic metastases. (B) Chest CT showing a small nodule of pulmonary metastasis. (C) Positron emission tomography (PET) showing multiple hepatic and aortocaval, paraaortic, and both common iliac lymph node metastases. (D) Whole-body bone scan showing an active bony lesion in L4.
References
1. Shin JS, Choi HJ, Choi YS, Chai SE, Choi HY. Predictive factor for the early progression of androgen independent prostate cancer in intermittent androgen deprivation therapy. Korean J Urol. 2004. 45:858–864.
2. Leibovici D, Spiess PE, Agarwal PK, Tu SM, Pettaway CA, Hitzhusen K, et al. Prostate cancer progression in the presence of undetectable or low serum prostate-specific antigen level. Cancer. 2007. 109:198–204.
3. Yamamoto S, Ito T, Akiyama A, Aizawa T, Miki M, Tachibana M. M1 prostate cancer with a serum level of prostate-specific antigen less than 10 ng/mL. Int J Urol. 2001. 8:374–379.
4. Namiki M, Ueno S, Kitagawa Y, Konaka H, Mizokami A, Koh E, et al. Hormonal therapy. Int J Clin Oncol. 2007. 12:427–432.
5. Na KI, Yoon DK, Cho JH. The change of serum prostate specific antigen after hormonal therapy of metastatic prostatic cancer. Korean J Urol. 1996. 37:1234–1238.
6. Sella A, Konichezky M, Flex D, Sulkes A, Baniel J. Low PSA metastatic androgen-independent prostate cancer. Eur Urol. 2000. 38:250–254.
7. Nishio R, Furuya Y, Nagakawa O, Fuse H. Metastatic prostate cancer with normal level of serum prostate-specific antigen. Int Urol Nephrol. 2003. 35:189–192.
8. Magklara A, Scorilas A, Stephan C, Kristiansen GO, Hauptmann S, Jung K, et al. Decreased concentrations of prostate-specific antigen and human glandular kallikrein 2 in malignant versus non-malignant prostatic tissue. Urology. 2000. 56:527–532.
9. Oefelein MG, Smith N, Carter M, Dalton D, Schaeffer A. The incidence of prostate cancer progression with undetectable serum prostate specific antigen in a series of 394 radical prostatectomies. J Urol. 1995. 154:2128–2131.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download