Abstract
Purpose
In this study, we report our initial experience with robot-assisted laparoscopic partial cystectomy (RLPC) in urachal diseases.
Materials and Methods
Two men and two women with a mean age of 51.5±9.3 years underwent RLPC between June 2009 and December 2009. In each case, a single surgeon using the da Vinci-S robotic system (Intuitive Surgical, Sunnyvale, CA, USA) used a transperitoneal approach with a 0° robotic camera. After careful observation of the intravesical portion of the mass, the mass was excised by use of monopolar scissors circumferentially. The bladder was closed in two layers with watertight running sutures made with 2-0 Vicryl.
Results
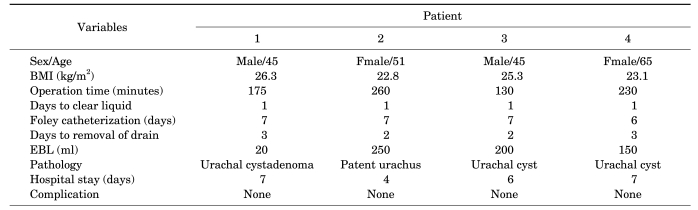
The mean operative time was 198 minutes (range, 130-260 minutes), the mean console time was 111 minutes (range, 70-150 minutes), and the mean estimated blood loss was 155 ml. The urethral catheter was removed on postoperative day 7 after a normal cystogram, and the surgical drain was removed on postoperative day 2.5 (range, 2-3 days). The mean hospital stay was 6 days (range, 4-7 days). There were no major complications. The pathology report revealed that one patient had a urachal cystadenoma, two patients had a urachal cyst, and one patient had a patent urachus.
Partial cystectomy is a bladder-preserving operation that has been utilized in both benign and malignant diseases. It is indicated in patients who have good bladder capacity and in cases of a solitary tumor in the bladder dome, a urachal tumor, or a tumor within the diverticulum with no concomitant carcinoma in situ (CIS) [1,2]. The advantages of partial cystectomy are minimizing the complications related to radical cystectomy in addition to preserving bladder function and erectile function [3]. Laparoscopic partial cystectomy is performed in cases of not only benign bladder masses, such as leiomyoma, pheochromocytoma, and endometriosis, but also in cases of malignant bladder tumors and urachal remnants [4-6]. More recently, robot-assisted laparoscopic surgery has emerged in the urology field, especially in the treatment of prostate cancer and bladder cancer [7,8]. In parallel with the positive results of robot-assisted laparoscopic prostatectomy and robot-assisted laparoscopic radical cystectomy, we anticipated similar benefits, including decreased morbidity, shortened operative time, and shortened hospital stay, from performing robot-assisted laparoscopic partial cystectomy (RLPC) on urachal diseases.
The urachus is a three-layered embryological structure that connects the allantois to the fetal urinary bladder. The urachus changes into a fibrous band after birth. Sometimes this regression may be incomplete and the urachal remnant may persist into adulthood. Various urachal abnormalities, such as urachal sinus, urachal cyst, vesicourachal diverticulum, and patent urachus, may lead to abdominal pain, umbilical drainage, or urachal cancer [9-11]. The incidence of urachal anomalies is very low. Drawson et al reported 2 cases in 300,000 admitted patients [12]. Because of the poor prognosis of urachal cancer with aging, urachal excision with partial cystectomy should be performed at diagnosis [9]. Herein, we report our initial experience of 4 cases of RLPC performed on urachal diseases and evaluate the perioperative and pathologic results.
Four patients underwent RLPC between June 2009 and December 2009 by a single surgeon using the da Vinci-S robotic system (Intuitive Surgical, Sunnyvale, CA, USA). Two men and two women, with a mean age of 51.5±9.3 years and a mean body mass index (BMI) of 24.4±1.7 kg/m2 underwent the operation. Each patient presented with gross hematuria, dysuria, and mucous secretion in the urine.
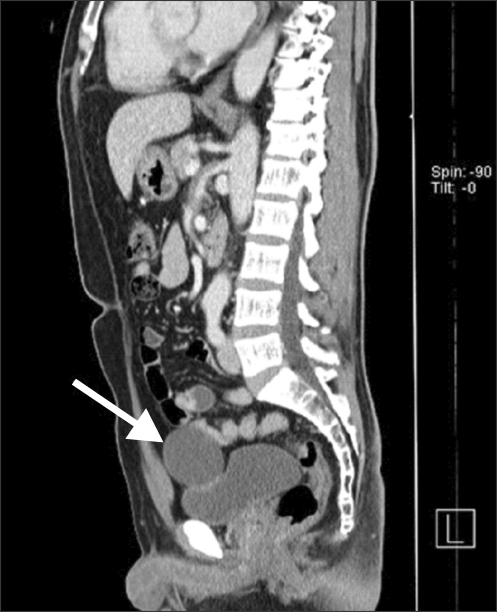
Patient 1 had hypertension and patient 2 had a history of trans-abdominal hysterectomy. The other patients had no specific medical or surgical history. Preoperative abdominal computed tomography (CT) scans revealed a solid mass in the dome area of the urinary bladder (Fig. 1). The clinical results of the RLPC were evaluated retrospectively.
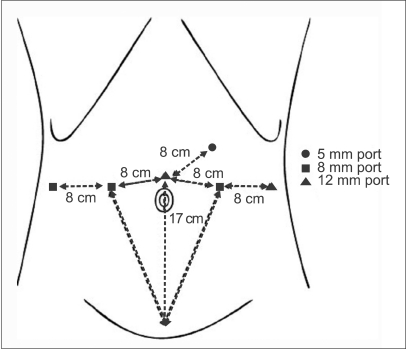
All patients underwent bowel preparation 1 day before surgery with an osmotic laxative. The patient was placed in the lithotomy position with both arms fixed against the body under general anesthesia. An 18 Fr. Foley catheter was inserted in the urethra, and pneumoperitoneum with carbon dioxide was established by using a Veress needle at a point 2 cm superior to the umbilicus. The transperitoneal approach was performed with six ports. The placement of the trocars is similar to that in robot-assisted laparoscopic radical prostatectomy but 2 cm superior to ensure that the urachus is accessible [13]. The 12 mm trocar was placed 2 cm superior to the umbilicus. The first robotic arm 8 mm trocar was placed 8 cm left laterally and the second robotic arm 8 mm trocar was placed 8 cm right laterally to the 12 mm camera port. The third robotic arm 8 mm trocar was placed 8 cm laterally to the second robotic arm port. The 12 mm assistant port was placed 8 cm laterally to the first robotic arm port, and the 5 mm assistant suction port was placed 8 cm superior to the camera port and the first robotic arm port (Fig. 2). After port placement, the patient was placed in the 30° Trendelenburg position and the robotic system was docked to the patient. The urinary bladder was filled with 200 cc of air, and a transperitoneal approach was performed with the 0° robotic camera with the use of monopolar scissors and a bipolar Maryland dissector. Any bowel adhesions were lysed with the monopolar scissors and the medial umbilical ligament was dissected. The bladder was released from the surrounding structure to permit identification of the bladder margins. After the cranial dissection into Retzius' space, the mass of the dome site was identified, and a cystotomy was performed with the monopolar scissors at a distance of 2 cm from the margin of the mass (Fig. 3). After careful observation of the intravesical portion of the mass, the mass was excised and removed by use of an EndoCatch® device. The bladder was closed in two layers with watertight running sutures made with 2-0 Vicryl. The bladder was filled with saline to detect any points of leakage. A Jackson-Pratt drain was positioned in the Retzius' space, and the specimen was removed from the camera port. A nasogastric tube was removed 1 day after surgery, and an oral liquid diet was started simultaneously.
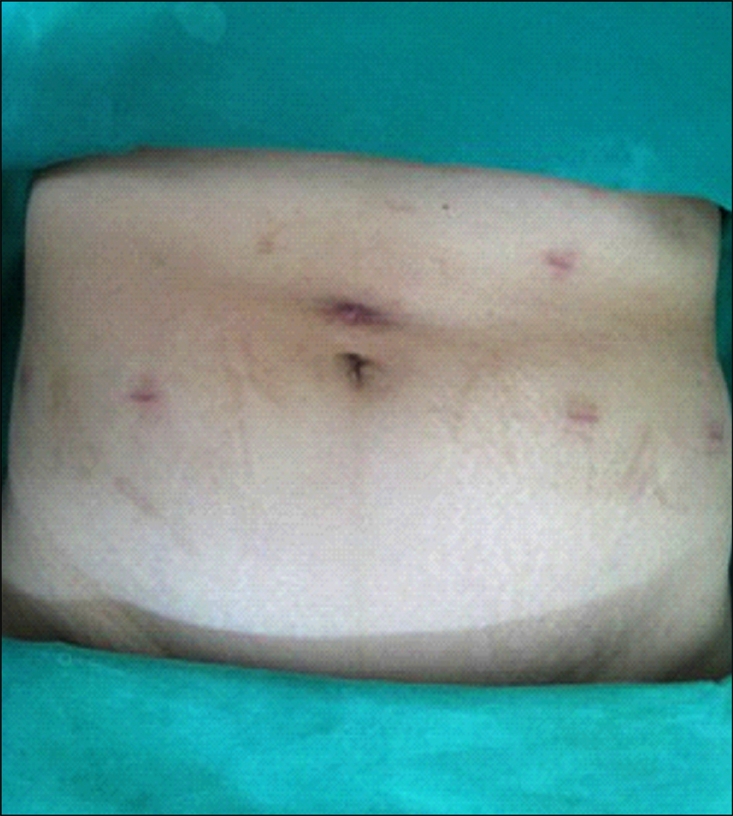
The mean operative time, including trocar placement as well as robotic docking and closure, was 198 minutes (range, 130-260 minutes). The mean console time was 111 minutes (range, 70-150 minutes). The mean estimated blood loss was 155 ml. There were no conversions to open surgery. The mean hospital stay was 6 days (range, 4-7 days). Drain removal was performed at postoperative day 2.5 (range, 2-3 days). Each patient underwent postoperative cystography on day 7 postoperatively, and no patients had evidence of extravasation. Two patients had the Foley catheter removed at that time, and the other two patients had the Foley catheter removed as outpatients after discharge on postoperative day 7. There were no major complications. A 1-month postoperative image of the port site is presented in Fig. 4. The operative results and the final pathology report of each case are presented in Table 1. Two patients were diagnosed with a urachal cyst, whereas the other two patients were diagnosed with patent urachus and urachal cystadenoma, respectively.
In the urology field, Rassweiler et al initially performed RALP in 2001 with the da Vinci™ robotic system [14]. Recently, robot-assisted laparoscopic prostatectomy has been performed worldwide, and it can overcome the limitations of open radical prostatectomy and laparoscopic radical prostatectomy. Many urologists have expressed interest in robotic surgery because of its comparable perioperative results to open surgery and conventional laparoscopic surgery, which have steep learning curves. After the application of a robot-assisted laparoscopic approach to prostate cancer, various operations have been successfully performed by use of robotic systems. To date, robot-assisted laparoscopic radical cystectomy has been performed in many medical centers [8]. The advantages of robot-assisted laparoscopic radical cystectomy include easier dissection and mobilization, which RLPC can also obtain. Our experience with robotic surgery for prostate cancer, kidney cancer, and ureteropelvic junction stricture acclimmated us to controlling the instruments and practicing suture techniques before performing RLPC. RLPC was initially performed in cases of bladder diverticulum [15,16]. Myer et al reported their initial experiences of 5 serial cases of robot-assisted bladder diverticulectomy, and a similar report was published by Meeks et al about robot-assisted bladder diverticulectomy in pediatric patients [15,16]. According to those reports, perioperative surgical outcomes were comparable with those of conventional laparoscopic and open diverticulectomy. Recently, Tareen et al reported their initial experiences with robot-assisted laparoscopic partial cystectomy and diverticulectomy assisted by simultaneous transurethral incision [17]. In the report of Tareen et al, partial cystectomy was performed in 2 cases and diverticulectomy was performed in 2 cases [17]. Tareen et al reported the feasibility of RLPC and the advantages of the precise suture technique and enhancement of surgeon visibility. In our study, RLPC was performed by a single surgeon using a transperitoneal approach. In the case of a dome area mass, a transperitoneal approach can facilitate access and permit a larger working space. The advantages of the preperitoneal approach are minimizing intra-abdominal complications and preventing intra-abdominal spillage [8]. The use of a transperitoneal approach to secure the margin of the excision site and to suture the excision site of the bladder is superior to the use of a preperitoneal approach, however. In performing partial cystectomy, making an incision with adequate surgical margins and minimizing the normal bladder excision is important for oncologic and functional outcomes. We detected the location of the mass by inflation and deflation with air. After detection of the lesion, we made a 1 cm incision at a 2 cm distance from the lesion. After the robotic camera was inserted into the incision site, the bladder lesion was detected and an adequate surgical margin was secured. The dome site lesion was excised with a 2 cm safety margin under robotic vision by use of monopolar scissors. In our cases, an en bloc excision with umbilectomy was not performed because of the small size of the mass with no evidence of lymphadenopathy in the preoperative CT scan or in intraoperative findings. However, the urachus, involving the median umbilical ligament, medial umbilical ligaments, and bladder dome, was excised. If oncologically indicated, an en bloc excision and pelvic lymphadenectomy could be performed completely by robot-assisted surgery. The bladder was closed in two layers in a watertight running fashion with 2-0 Vicryl. The mobilization of the bladder and the suturing were done more efficiently by use of the robotic instruments, lowering the suturing time to less than 30 minutes. Tareen et al reported their initial experiences with robot-assisted laparoscopic partial cystectomy and diverticulectomy, and their mean operative duration was 190 minutes and the estimated blood loss was 35 ml; these results were similar to ours [17].
With regard to pathologic results, urachal cyst, patent urachus, and urachal cystadenoma were diagnosed in our cases. Patent urachus and urachal cyst can present with numerous signs such as drainage of fluid from the umbilicus, calculus formation in cysts, abdominal pain due to cystic hemorrhage, and peritonitis [3]. Urachal cystadenoma is a rarely reported pathology that can cause a palpable mass and abdominal pain. An aggressive clinical course can occur with the development of pseudomyxoma peritonei [18]. In our study, adult patients over 40-years-old were included. Adult patients with urachal anomalies should undergo an abdominal CT scan because of the high risk of malignancy of up to 25%. Ashley et al reported the increased risk of malignancy of a cystic urachal mass with increasing age [9]. In their study, 20% of patients with urachal cancer presented with metastatic disease. Therefore, early surgical treatment is mandatory in aged patients with urachal diseases.
Although open partial cystectomy and laparoscopic partial cystectomy have been treatments of choice for many years, our perioperative results of RLPC were comparable with those of laparoscopic partial cystectomy for urachal adenocarcinoma [19]. The robot-assisted laparoscopic approach in partial cystectomy is an attractive surgical alternative because of its association with superior surgical management with 3-dimensional magnified visualization and 7 degrees of articulation, which leads to fine resection and delicate intracorporeal suturing for anastomosis of the bladder with minimal difficulty. The disadvantages of a robotic system are its relatively high cost, lack of tactile feedback, additional operation time for the docking procedure, and additional port placement compared with conventional laparoscopic partial cystectomy [20]. Considering our initial experience with these 4 cases of RLPC, after sufficient experience, the time for the docking procedure can be minimized, and the number of ports placed can be reduced to 4 or 5 ports by excluding the 5 mm assistant port and the third arm port. Despite several disadvantages, the superior surgical management of the robotic system enables the surgeon to perform a delicate operation in a tight pelvic cavity. A randomized controlled study comparing RLPC with conventional laparoscopic partial cystectomy or open partial cystectomy is not feasible because of the rarity of benign urachal diseases. However, this initial experience demonstrates the safety and feasibility of RLPC.
Our initial clinical experience suggests that RLPC is a feasible and safe treatment modality for patients with benign urachal diseases. The perioperative surgical outcomes offer encouraging results that make RLPC a reasonable surgical alternative. However, more cases are required to confirm the efficacy of RLPC.
References
1. Nieh PT, Marshall FF. Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Surgery of bladder cancer. Campbell-Walsh urology. 2007. 9th ed. Philadelphia: Saunders;p. 2503–2505.
2. Holzbeierlein JM, Lopez-Corona E, Bochner BH, Herr HW, Donat SM, Russo P, et al. Partial cystectomy: a contemporary review of the Memorial Sloan-Kettering Cancer Center experience and recommendations for patient selection. J Urol. 2004; 172:878–881. PMID: 15310988.

3. Kim BH, Kim JH, Park WJ, Ryu DS, Kwon JO, Oh TH. Laparoscopic partial cystectomy for adenocarcinoma of the bladder. Korean J Urol. 2007; 48:990–993.

4. Tai HC, Chung SD, Wang SM, Chueh SC, Yu HJ. Laparoscopic partial cystectomy for various bladder pathologies. BJU Int. 2007; 100:382–385. PMID: 17506869.

5. Geol H, Kim DW, Kim TH, Seong YK, Cho WY, Kim SD, et al. Laparoscopic partial cystectomy for schwannoma of urinary bladder: case report. J Endourol. 2005; 19:303–306. PMID: 15865518.

6. Colombo JR Jr, Desai M, Canes D, Frota R, Haber GP, Moinzadeh A, et al. Laparoscopic partial cystectomy for urachal and bladder cancer. Clinics. 2008; 63:731–734. PMID: 19060992.

7. Ficarra V, Cavalleri S, Novara G, Aragona M, Artibani W. Evidence from robot-assisted laparoscopic radical prostatectomy: a systematic review. Eur Urol. 2007; 51:45–55. PMID: 16854519.

8. Sala LG, Matsunaga GS, Corica FA, Ornstein DK. Robot-assisted laparoscopic radical cystoprostatectomy and totally intracorporeal ileal neobladder. J Endourol. 2006; 20:233–235. PMID: 16646646.

9. Ashley RA, Inman BA, Routh JC, Rohlinger AL, Husmann DA, Kramer SA. Urachal anomalies: a longitudinal study of urachal remnants in children and adults. J Urol. 2007; 178:1615–1618. PMID: 17707039.

10. Yiee JH, Garcia N, Baker LA, Barber R, Snodgrass WT, Wilcox DT. A diagnostic algorithm for urachal anomalies. J Pediatric Urol. 2007; 3:500–504.

11. Cilento BG Jr, Bauer SB, Retik AB, Peters CA, Atala A. Urachal anomalies: defining the best diagnostic modality. Urology. 1998; 52:120–122. PMID: 9671882.

12. Drawson JS, Crisp AJ, Boyd SM, Broderick NJ. Case report: benign urachal neoplasm. Br J Radiol. 1994; 67:1132–1133. PMID: 7820408.
13. Park SY, Ham WS, Choi YD, Rha KH. Robot-assisted laparoscopic radical prostatectomy: clinical experience of 200 cases. Korean J Urol. 2008; 49:215–220.

14. Rassweiler J, Binder J, Frede T. Robotic and telesurgery: will they change our future? Curr Opin Urol. 2001; 11:309–320. PMID: 11371786.

15. Myer EG, Wagner JR. Robotic assisted laparoscopic bladder diverticulectomy. J Urol. 2007; 178:2406–2410. PMID: 17937944.

16. Meeks JJ, Hagerty JA, Lindgren BW. Pediatric robotic-assisted laparoscopic bladder diverticulectomy. Urology. 2009; 73:299–301. PMID: 18817959.
17. Tareen BU, Mufarrij PW, Godoy G, Stifelman MD. Robot-assisted laparoscopic partial cystectomy and diverticulectomy: initial experience of four cases. J Endourol. 2008; 22:1497–1500. PMID: 18690815.

18. Schell AJ, Nickel CJ, Isotalo PA. Complex mucinous cystadenoma of undetermined malignant potential of the urachus. Can Urol Assoc J. 2009; 3:E39–E41. PMID: 19672436.

19. Wadhwa P, Kolla SB, Hemal AK. Laparoscopic en bloc partial cystectomy with bilateral pelvic lymphadenectomy for urachal adenocarcinoma. Urology. 2006; 67:837–843. PMID: 16618570.

This article describes the authors' experience with four cases of robot-assisted laparoscopic partial cystectomy in urachal diseases in a 7-month period. It is only recently that robotic technology has been utilized in partial cystectomy [1-3]. This technology offers both surgical efficacy and the minimally invasive benefits of robotic surgery as evidenced by experiences with robotic prostatectomy and robotic cystectomy (authors' references 13, 20). However, there is always the chance of malignancy in urachal disease, which should always be kept in mind. Furthermore, because of the added cost of using a surgical robot, this method should be reserved for more complex cases, such as large defects or more posterior or distally located masses for which the conventional laparoscopic approach may be a challenge.
References
1. Spiess PE, Correa JJ. Robotic assisted laparoscopic partial cystectomy and urachal resection for urachal adenocarcinoma. Int Braz J Urol. 2009; 35:609. PMID: 19860941.

2. Allaparthi S, Ramanathan R, Balaji KC. Robotic partial cystectomy for bladder cancer: a single-institutional pilot study. J Endourol. 2010; 24:223–227. PMID: 20039797.

3. Pandey R, Garg R, Roy K, Darlong V, Punj J, Kumar A. Perianesthetic management of the first robotic partial cystectomy in bladder pheochromocytoma. A case report. Minerva Anestesiol. 2010; 76:294–297. PMID: 20332744.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download