Abstract
Purpose
We aimed to investigate the significance of early detection of transition zone prostate cancer by transurethral resection of prostate (TURP) in benign prostatic hyperplasia (BPH) patients with lower urinary tract symptoms (LUTS) in whom prostate cancer was suspected despite a negative transrectal ultrasonography (TRUS) biopsy result.
Materials and Methods
From January 2006 to January 2009, a total of 165 patients who underwent TURP were evaluated. The prostate cancer detection rate was compared between patients who underwent TRUS biopsy before TURP (group A) and those who did not (group B). All charts were evaluated retrospectively, including prostate-specific antigen (PSA), digital rectal examination (DRE), TURP results (including resection volume and pathology report), TRUS, and TRUS biopsy results. Group A was subdivided into group A1, who were diagnosed with prostate cancer after TURP, and group A2, who were diagnosed with BPH after TURP.
Results
The cancer detection rate showed no significant difference between groups A and B (8.9% vs. 7.5%, p>0.05). The mean PSA levels in groups A1 and A2 were 15.5±14.0 ng/ml and 9.1±5.1 ng/ml, respectively (p>0.05). In group A1, 40% had an abnormal DRE, compared with 6.7% in group A2 (p<0.05). After TURP, the mean percentage of resected prostatic chips of the prostate cancer group and BPH group were 33.9% and 18.6%, respectively (p=0.001). A positive correlation was found between the detection rate of prostate cancer and PSA (p=0.01).
Conclusions
BPH patients in whom prostate cancer is suspected and who have lower urinary tract symptoms (LUTS) with a previously negative TRUS biopsy result can undergo TURP, which results in immediate improvement in bladder outlet obstruction and early diagnosis of clinically significant transition zone prostate cancer.
The incidence of prostate cancer is increasing in Korea owing to a westernization of dietary life. The detection rate of prostate cancer by transrectal ultrasonography (TRUS) biopsy has increased along with the utilization of both prostate-specific antigen (PSA) and the digital rectal examination (DRE) as screening tests for prostate cancer. In the PSA era, the detection rate of stage T1c prostate cancer has increased. The diagnosis rate of T1a and T1b stage prostate cancer was 12.9% in the pre-PSA era in the late 1980s. However, upon the introduction of PSA, the diagnosis rate of stage T1a and T1b prostate cancer decreased to 8.0% in the late 1990s. It is noteworthy that there was no significant difference in stage T1a prostate cancer, but there was a significant decrease in stage T1b prostate cancer [1].
When TRUS biopsy is performed because of an elevated PSA value, the detection rate of prostate cancer can vary according to factors such as the location of the cancer in the prostate, the number of cores, and the method of TRUS biopsy. The detection rate of prostate cancer by TRUS biopsy by randomized sextant core has been reported to be 25-30%; the detection rates of prostate cancer by sextant biopsy with meticulous focus on the far lateral portion and that by 10-core and 12-core biopsy have been reported and advocated by many institutes [2,3]. With an increased number of biopsy cores, morbidity such as rectal bleeding, hematuria, fever, and pain does not increase significantly and unnecessary repeat biopsy is decreased [3]. Even with a negative result on an initial TRUS biopsy, repetition of TRUS biopsy is recommended when clinical suspicion of prostate cancer exists, such as with PSA elevation or an abnormal DRE [4]. However, it is difficult to detect prostate cancer by repeat TRUS biopsy when the tumor is located in the transition zone. Thus, transurethral biopsy or transurethral resection of prostate (TURP) are the preferred approach in selected cases [4]. Transition zone prostate cancer is detected mostly on the anterior part of the prostate and is hard to reach by TRUS biopsy [5]. The aim of this study was to evaluate the significance of early detection of transition zone prostate cancer by TURP in benign prostatic hyperplasia (BPH) patients with lower urinary tract symptoms (LUTS) in whom prostate cancer was suspected despite a negative TRUS biopsy result.
From January 2006 to January 2009, a total of 165 BPH patients with LUTS who underwent TURP were studied. TRUS biopsy was performed in those patients whose PSA was over 4 ng/ml or who had an abnormal nodule on the DRE. Patients with histologically confirmed prostate cancer by TRUS biopsy were excluded from this study. Regardless of TRUS biopsy, patients who had bladder outlet obstruction with LUTS underwent TURP to improve their LUTS, and TURP was performed 1 month after TRUS biopsy. The 12-core TRUS biopsy (including a 2-core transition zone biopsy) protocol was performed, and additional cores were sampled at hypoechoic lesions observed on the TRUS image. All patients' charts were evaluated retrospectively, including International Prostate Symptom Score (IPSS), PSA, DRE, TURP results (including resection volume and pathology report), TRUS, and TRUS biopsy results.
Patients who underwent TRUS biopsy were classified into group A, and those who did not undergo TRUS biopsy were classified into group B. The detection rate of prostate cancer was compared bewteen the two groups. Group A was subdivided into group A1, who were diagnosed with prostate cancer after TURP, and group A2, who were diagnosed with nodular hyperplasia after TURP.
IPSS/quality of life (QoL), PSA, DRE, TRUS, prostate volume, transition zone volume, and PSA density were compared between group A1 and group A2. Patients in group A were classified and compared by age (≤65 years, 66-75 years, ≥75 years), DRE, and PSA (≤4 ng/ml, 4-10 ng/ml, ≥10 ng/ml). The clinical stage and Gleason's scores of patients who had diagnosed prostate cancer after TURP were analyzed. Statistical analysis was performed by exact chi-square test, Fisher's exact test, and linear-by-linear association with SPSS 13.0, and p-values of less than 0.05 were deemed significant.
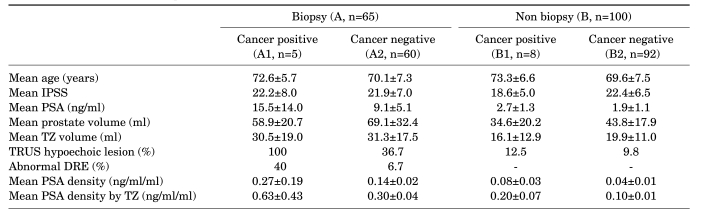
Prostate cancer was detected in 13 patients (7.9%), 8 of whom were diagnosed with stage T1a (4.8%) and 5 of whom were diagnosed with stage T1b (3%). Group A consisted of 65 patients who had undergone TRUS biopsy before TURP, and 5 of those patients (7.7%) were diagnosed with prostate cancer. Among group B, 8 patients were diagnosed with prostate cancer (8.0%) (Table 1).
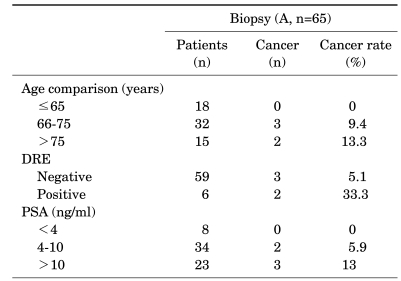
In group A, the mean ages of group A1 and group A2 were 72.6±5.7 years (median: 70 [range, 66-80]) and 70.1±7.3 years, respectively. There was no significant difference in age between the two groups (p=0.458) (Table 1). The IPSS of group A1 and group A2 were 22.2±8.0 (median: 24 [range, 11-32]) and 21.9±7.0, respectively (p=0.092) (Table 1). The mean PSA of group A1 (15.5±14.0 ng/ml; median: 7.3 [range, 3.1-32.4]) was higher than that of group A2 (9.1±5.1 ng/ml), but there was no significant difference between the two groups (p=0.368) (Table 1). The mean volume of the total prostate was 58.9±20.7 ml (median: 62.6 [range, 34.7-88.8 ml]) in group A1 and 69.1±32.4 ml in group A2 (p=0.493) (Table 1). The mean volume of the transition zone was 30.5±19.0 ml (median: 23.3 [range, 8.0-55.5 ml]) in group A1 and 31.3±17.5 ml in group A2 (p=0.750) (Table 1). There was a significant difference in the detection rate of hypoechoic lesions by TRUS between group A1, for which the rate was 100% (p=0.01) (Table 1), and group A2, for which the rate was 36.7%. A total of 40% of the patients in group A1 showed abnormal nodules on the DRE, whereas 6.7% of patients in group A2 showed abnormal nodules on the DRE (p=0.063) (Table 1). Mean PSA density was 0.27±0.19 ng/ml/ml in group A1 (median: 0.16 [range, 0.03-0.52 ng/ml/ml]) and 0.14±0.02 ng/ml/ml in group A2 (p=0.237) (Table 1). Mean PSA density by transition zone was 0.63±0.43 ng/ml/ml in group A1 (median: 0.67 [range, 0.06-1.32 ng/ml/ml]) and 0.30±0.04 ng/ml/ml in group A2 (p=0.211) (Table 1). After TURP, the mean percentages of resected prostatic chips of the prostate cancer group and the BPH group were 33.9% and 18.6%, respectively (p=0.001).The detection rate of prostate cancer showed no significant difference with age (p=0.351, linear-by-linear association=0.198) (Table 2) or DRE (p=0.063) (Table 2). A positive correlation was found between the detection rate of prostate cancer and PSA (p=0.01) (Table 2).
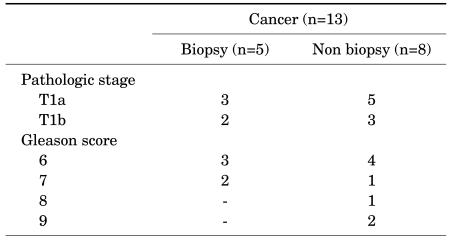
Among 13 patients who were diagnosed with prostate cancer after TURP, 5 patients underwent TRUS biopsy before TURP, and the remaining patients did not undergo TRUS biopsy (Table 3).
On the basis of clinical staging, of 5 patients who had undergone TRUS biopsy before TURP, 3 patients (4.6%) were classified as having stage T1a cancer and 2 patients (3.1%) as having stage T1b. Of 8 patients who had not undergone TRUS biopsy before TURP, 5 patients (5.0%) were classified as having stage T1a cancer and 3 patients (3.0%) as having stage T1b (p=1.000) (Table 3).
On the basis of Gleason's score, among the patients who had undergone TRUS biopsy before TURP, 3 patients (60%) were classified as having a Gleason's score of 6 and 2 patients (40%) as having a Gleason's score of 7. Among those who had not undergone TRUS biopsy before TURP, 4 patients (50%) were classified as having a Gleason's score of 6, 1 patient (12.5%) as having a Gleason's score of 7, 1 patient (12.5%) as having a Gleason's score of 8, and 2 patients (25%) as having a Gleason's score of 9 (p=0.641) (Table 3).
Patients without TRUS biopsy before TURP showed higher Gleason's scores, and clinically high-grade Gleason's scores, including Gleason's scores over 7, were detected in several cases, but there was no statistically significant difference between the two groups. The patients who were diagnosed with prostate cancer after TURP were treated by active surveillance.
Despite the recent introduction of several minimally invasive surgical methods of treating BPH, TURP has been recognized as the gold standard in the treatment of BPH. TURP is safe and feasible even in a large prostate, and it can replace open prostatectomy [6]. Bipolar TURP has been proven to be a safe and effective treatment alternative [7,8], leading to frequent application of TURP, even in large prostates. To the best of our knowledge, however, the detection rate of prostate cancer after TURP has rarely been addressed in published reports [9].
A diagnosis of stage T1a and T1b prostate cancer is made through pathologic examination of resected prostatic tissue specimens from TURP. Otherwise, pathologic stages of T0, T2, or over T2 can be proved only after total excision of the prostate. The detection rate of stage T1a and T1b prostate cancer has been decreasing, mostly for the following reasons. First, TURP has largely been replaced by pharmacologic medication. Second, PSA is generally used as a screening test in patients with LUTS. Therefore, patients who should have been diagnosed with stage T1a-T1b are now diagnosed with stage T1c prostate cancer instead. Tombal et al reported that among patients who had undergone surgical intervention, the rate of T1a prostate cancer decreased from 23% to 7% and that of T1b prostate cancer decreased from 15% to 2% between 1985 and 1997 [10,11]. Mai et al also reported that the proportion of stage T1a and T1b prostate cancer was 12.9% between 1989 and 1990, but that it decreased to 8.0% between 1997 and 1999 [1]. In our study, 13 patients (7.9%) were diagnosed with prostate cancer after TURP among the total 165 patients. Patients with T1a and T1b cancer numbered 8 (4.8%) and 5 (3.0%), respectively. Our results were similar to previously reported data [1].
In cases clinically suspicious of prostate cancer, TRUS biopsy is usually recommended. Recently, compared with sextant biopsy, extended biopsy such as 8-core biopsy, 12-core biopsy, and more laterally oriented biopsy has been reported to provide an increased detection rate of prostate cancer [12].
In cases of persistently high PSA, the detection rate of prostate cancer can rise from 77% in the first TRUS biopsy to 99% in the fourth biopsy with repeated TRUS biopsy [13]. However, TRUS biopsy is an invasive procedure that can cause complications such as infection, hemorrhage, and sepsis. Roehl et al reported a significant decrease in the prostate cancer detection rate to 10% if the results of the initial two TRUS biopsies are concluded to be negative [14]. Several reports have suggested the necessity of TURP to diagnose transition zone prostate cancer in patients with persistent elevation of PSA and repetitive negative TRUS biopsy results. Puppo et al reported that performing TURP and lateralized TRUS biopsy in patients with 3 or more previous negative TRUS biopsies can raise the detection rate up to 57% [6]. Recently, repetitive negative prostate cancer results were evaluated with extended biopsy and TURP. In cases with a history of 2 or more negative TRUS biopsies, extended saturation biopsy and concomitant TURP showed a reasonable diagnostic yield and relief of obstruction [15]. The use of TURP as a diagnostic tool was founded by the fact that most transition zone prostate cancer is located at the anterior part of the prostate, which is difficult to approach by TRUS biopsy [5]. Steuber et al reported the different biological characteristics of transition zone cancer, which shows a lower grade; additionally, in radical prostatectomy specimens, satellite tumors were noted with higher grades in the peripheral zone [16]. In our study, 2 transition zone biopsy cores were included in the TRUS biopsy protocol. In cases of BPH, especially in prostates of over 50 g, the peripheral zone is compressed and thinned by hyperplasia of the transition zone. Therefore, biopsy cores that target the medial peripheral zone can include the transition zone as well as the peripheral zone, thus lowering the necessity for additional transition zone biopsies [17]. Therefore, in cases of BPH patients who complain of severe LUTS and with a clinical suspicion of prostate cancer after negative results of a 12-core TRUS biopsy with no high-grade prostatic intraepithelial neoplasia (PIN), atypical small acinar proliferation (ASAP), or persistent elevation of PSA, immediate TURP is a reasonable approach owing to the rapid relief of bladder outlet obstruction and feasible diagnosis of transition zone prostate cancer.
Concerning the treatment of prostate cancer after TURP, Puppo et al reported that previous TURP had no negative effects on the outcomes of radical prostatectomy. In a previous study, radical prostatectomy after TURP was reported to be more difficult because of worsened conditions for radical retropubic prostatectomy (RRP), such as periprostatic fibrotic tissue, bladder neck injury, and capsular violation [18]. However, in recent reports, perioperative morbidity and functional results, such as continence and erectile function, in open RRP after TURP were comparable with those after RRP alone with no previous TURP [19]. Recently, laparoscopic radical prostatectomy has been performed after TURP. Although it is a challenging procedure owing to the distortion of the surgical planes, it was oncologically safe and overall potency rates were preserved [20].
In our study, there was no significant difference in the detection rate of prostate cancer between the prostate cancer suspicious group (8.9%) and the nonsuspicious group (7.5%). Our pathologic results showed 1 patient (12.5%) who had a Gleason's score of 8 and 2 patients (25%) who had a Gleason's score of 9. These results suggest that a relatively high rate of clinically significant tumors were detected by TURP, and prostate cancer that was detected by TURP could be treated surgically, such as by RRP or laparoscopic radical prostatectomy in selective cases.
Kitamura et al suggested that TURP should be performed only in patients who complain of LUTS, because most clinically nonsignificant prostate cancer is diagnosed by performing concomitant sextant biopsy and transition zone biopsy [21]. However, van Renterghem et al reported improvement of bladder outlet obstruction, normalization of PSA, and diagnosis of clinically significant prostate cancer by performing TURP [22].
In our study, the proportion of stage T1a and of stage T1b prostate cancer was not significantly different between groups A and B. These data can lead to the conclusion that TURP does not significantly influence clinical staging in T1a, T1b transition zone prostate cancer. Thus, performing TURP in patients with previously negative TRUS biopsy results who are clinically suspicious for prostate cancer would not significantly influence the treatment of prostate cancer. Furthermore, most transition zone prostate cancer was located at the anterior part of the prostate, which is difficult to approach by TRUS biopsy. Approach to the transition zone is better by TURP, which could relieve bladder outlet obstruction and detect transition zone prostate cancer.
The clinical stage of T1a, T1b prostate cancer was similar between the prostate cancer suspicious BPH group and the nonsuspicious group. Also, even with 12-core TRUS biopsy, which included 2-core biopsy of the transition zone, precise access to the transition zone is troublesome. Therefore, BPH patients with a clinical suspicion of prostate cancer who have LUTS and a previously negative TRUS biopsy result can undergo TURP, which results in immediate improvement of the bladder outlet obstruction and early diagnosis of clinically significant transition zone prostate cancer.
References
1. Mai KT, Isotalo PA, Green J, Perkins DG, Morash C, Collins JP. Incidental prostatic adenocarcinomas and putative premalignant lesions in TURP specimens collected before and after the introduction of prostate-specific antigen screening. Arch Pathol Lab Med. 2000; 124:1454–1456. PMID: 11035574.
2. Cho KJ, Ha US, Lee CB. Role of transurethral resection of the prostate in the diagnosis of prostate cancer for patients with lower urinary tract symptoms and serum PSA 4-10 ng/ml with a negative repeat transrectal needle biopsy of prostate. Korean J Urol. 2007; 48:1010–1015.
3. Mariappan P, Chong WL, Sundram M, Mohamed SR. Increasing prostate biopsy cores based on volume vs the sextant biopsy: a prospective randomized controlled clinical study on cancer detection rates and morbidity. BJU Int. 2004; 94:307–310. PMID: 15291857.

4. O'dowd GJ, Miller MC, Orozco R, Veltri RW. Analysis of repeated biopsy results within 1 year after a non cancer diagnosis. Urology. 2000; 55:553–559. PMID: 10736500.
5. Eggener SE, Roehl KA, Catalona WJ. Predictors of subsequent prostate cancer in men with a prostate specific antigen of 2.6 to 4.0 ng/ml and an initially negative biopsy. J Urol. 2005; 174:500–504. PMID: 16006880.

6. Kwak DY, Chang HS, Park CH, Kim CI. Long-term results of transurethral resection of the prostate for large benign prostatic hyperplasia: a comparative study with open prostatectomy. Korean J Urol. 2008; 49:31–36.

7. Kim JY, Moon KH, Yoon CJ, Park TC. Bipolar transurethral resection of the prostate: a comparative study with monopolar transurethral resection. Korean J Urol. 2006; 47:493–497.

8. Kim HG, Lee BK, Paick SH, Lho YS. Efficacy of bipolar transurethral resection of the prostate: comparison with standard monopolar transurethral resection of the prostate. Korean J Urol. 2006; 47:377–380.

9. Puppo P, Introini C, Calvi P, Naselli A. Role of transurethral resection of the prostate and biopsy of the peripheral zone in the same session after repeated negative biopsies in the diagnosis of prostate cancer. Eur Urol. 2006; 49:873–878. PMID: 16439052.

10. Fowler JE Jr, Pandey P, Bigler SA, Yee DT, Kolski JM. Trends in diagnosis of stage T1a-b prostate cancer. J Urol. 1997; 158:1849–1852. PMID: 9334615.

11. Tombal B, De Visccher L, Cosyns JP, Lorge F, Opsomer R, Wese FX, et al. Assessing the risk of unsuspected prostate cancer in patients with benign prostatic hypertrophy: a 13-year retrospective study of the incidence and natural history of T1a-T1b prostate cancers. BJU Int. 1999; 84:1015–1020. PMID: 10571626.

12. Monda JM, Barry MJ, Oesterling JE. Prostate specific antigen cannot distinguish stage T1a (A1) prostate cancer from benign prostatic hyperplasia. J Urol. 1994; 151:1291–1295. PMID: 7512660.

13. Ahn HS, Kwon CH, Joo KJ. Comparative analysis of the prostate cancer detection rate according to region and number of biopsy in patient with elevated serum PSA. Korean J Urol. 2006; 47:591–595.

14. Roehl KA, Antenor JA, Catalona WJ. Serial biopsy results in prostate cancer screening study. J Urol. 2002; 167:2435–2439. PMID: 11992052.

15. Ploussard G, Dubosq F, Boublil V, Allory Y, de la Taille AD, Vordos D, et al. Extensive biopsies and transurethral prostate resection in men with previous negative biopsies and high or increasing prostate specific antigen. J Urol. 2009; 182:1342–1349. PMID: 19683310.

16. Steuber T, Karakiewicz PI, Augustin H, Erbersdobler A, Haese A, Chun KH, et al. Transition zone cancers undermine the predictive accuracy of Partin table stage predictions. J Urol. 2005; 173:737–741. PMID: 15711259.

17. Hwang SI, Lee HJ, Cho JY, Kim SH, Lee SE, Byun SS, et al. Should transition zone biopsies be added to 12-core systematic biopsies of the prostate? J Clin Ultrasound. 2009; 37:281–284. PMID: 19309725.

18. Djavan B, Mazal P, Zlotta A, Wammack R, Ravery V, Remzi M, et al. Pathological features of prostate cancer detected on initial and repeat prostate biopsy: results of the prospective European Prostate Cancer Detection study. Prostate. 2001; 47:111–117. PMID: 11340633.

19. Palisaar JR, Wenske S, Sommerer F, Hinkel A, Noldus J. Open radical retropubic prostatectomy gives favourable surgical and functional outcomes after transurethral resection of the prostate. BJU Int. 2009; 104:611–615. PMID: 19298408.

20. Teber D, Cresswell J, Ates M, Erdogru T, Hruza M, Gözen AS, et al. Laparoscopic radical prostatectomy in clinical T1a and T1b prostate cancer: oncologic and functional outcomes--a matched-pair analysis. Urology. 2009; 73:577–581. PMID: 19100598.

21. Kitamura H, Masumori N, Tanuma Y, Yanase M, Itoh N, Takahashi A, et al. Does transurethral resection of the prostate facilitate detection of clinically significant prostate cancer that is missed with systematic sextant and transition zone biopsies? Int J Urol. 2002; 9:95–99. PMID: 12028299.

22. van Renterghem K, Van Koeveringe G, Achten R, Van Kerrebroeck P. Clinical relevance of transurethral resection of the prostate in "asymptomatic" patients with an elevated prostate-specific antigen level. Eur Urol. 2007; 52:819–826. PMID: 17418482.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download