Abstract
Purpose
To investigate the learning curve for robot-assisted laparoscopic radical prostatectomy (RALP) for pathologic T2 disease, we examined differences in perioperative outcomes according to time period.
Materials and Methods
Between July 2005 and June 2008, a total of 307 consecutive patients underwent RALP for prostate cancer and 205 patients had pathologic T2 disease. Patients were grouped into 6-month time periods. We collected and examined the patient's perioperative data including age, body mass index (BMI), prostate-specific antigen (PSA), operation time, estimated blood loss, and positive surgical margin.
Results
There were no significant differences among the groups in age (p=0.705), BMI (p=0.246), PSA (p=0.425), or prostate volume (p=0.380). Operation time (p<0.001) and estimated blood loss (p<0.001) decreased significantly with time. The positive surgical margin rate also showed a decreasing trend, but this was not significant (p=0.680).
Recently, robotic systems have been introduced to various surgical fields, including urologic surgery. In 2001, Binder and Kramer performed the first robot-assisted laparoscopic prostatectomy (RALP) [1]. In 2005, the first domestic RALP was performed with the da Vinci™ robot system [2]. Cases of RALP are increasing abruptly as the robotic equipment becomes more widely used.
In general, robotic surgery has been considered to have a short learning curve in comparison with laparoscopic surgery. However, only a few reports have been published on the learning curve for RALP. Also, because most of the studies published previously described all patients who had undergone RALP, the inclusion of more patients with pathologic T3 disease over a certain period of time could result in selection bias that would negatively influence the results.
The purpose of this study was to investigate the learning curve of RALP for pathologic T2 disease by evaluating differences in perioperative outcomes according to time period.
Between July 2005 and June 2008, a total of 307 consecutive patients had undergone RALP for prostate cancer. Of those men, 205 patients with diagnosed pathologic T2 disease were enrolled in this study. The da Vinci™ robot system (Intuitive Surgical Inc., Sunnyvale, USA) was used from July 2005 through May 2007 and the da Vinci S™ robot system was used from June 2007 through June 2008. The total period of our study was 36 months, which was divided into 6-month periods. The numbers of patients who underwent RALP during each 6 months were 7, 17, 33, 49, 35, and 60 patients, respectively.
All patients had prostate cancer diagnosed by transrectal ultrasound-guided prostate needle biopsy. For staging a workup, whole-body bone scan and either abdominal computed tomography (CT) or prostate magnetic resonance imaging (MRI) were performed. The TNM 2002 classification was used for staging the operative specimens.
We collected all patients' data including perioperative clinical outcomes and short-term surgical results. Preoperative information collected included age, body mass index (BMI), prostate-specific antigen (PSA), and Gleason's score. Intraoperative information collected included operation time and estimated blood loss. Operation time was defined as the time from initial incision to the completion of the last skin suture described in the anesthesia report, and estimated blood loss was calculated by the anesthesiologist. The postoperative information collected included pathologic results, margin status, postoperative complications, duration of hospitalization, and duration of catheterization.
All surgeries were performed by only one surgeon by use of the transperitoneal approach. The ports for the da Vinci™ robot system and laparoscopic tools were placed and RALP was performed as previously reported [3]. Except for the initial 15 patients, all other patients underwent pelvic lymphadenectomy. Pelvic lymph node dissection was performed bilaterally in the external iliac, obturator, and infraobturator areas.
Statistical analysis was performed by using SPSS version 17.0 (SPSS Inc, Chicago, USA) by applying ANOVA, chi-square test, and Spearman's rank correlation test. Differences were considered statistically significant at p<0.05.
From July 2005 to June 2008, the mean age of the total 205 patients with diagnosed pathologic T2 prostate cancer after RALP was 63±8 years. The patients' mean BMI was 24.76±2.52 kg/m2. The preoperative mean PSA value was 12.72±50.77 ng/ml, and the average Gleason's score was 6.6 (range, 3-9). Mean prostate volume was 32.3±13.4 g. There were no significant differences in age (p=0.705), BMI (p=0.246), PSA (p=0.425), preoperative Gleason's score (p=0.201), or prostate volume (p=0.380) among the 6-month time periods.
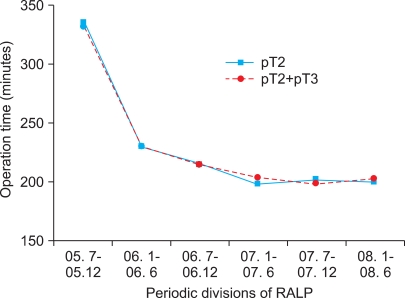
The overall mean operation time including robot installation and pelvic lymphadenectomy was 210±46 min, and mean operation time decrease as the number of cases increased (Spearman's rho -0.268, p<0.001). The mean operation time of each period was 335±64, 230±52, 215±38, 199±31, 202±28, and 200±43 min, respectively, and there was a significant difference between the first period and the second to sixth periods (p<0.001). However, there were no significant differences among the second to sixth periods (Fig. 1).
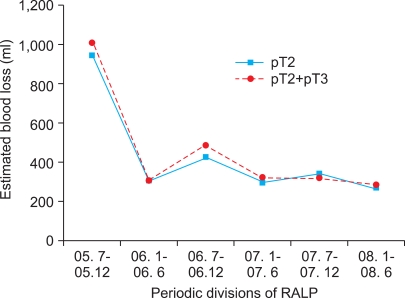
The mean volume of estimated blood loss was 337±287 ml, and estimated blood loss also decreased as the number of cases increased (Spearman's rho -0.188, p<0.007). The mean estimated blood loss of each 6-month period was 943±988, 302±164, 424±240, 295±200, 336±229, and 266±62 ml, respectively. Similar to the mean operation time, there was a significant difference between the first period and the second to sixth periods (p<0.001), but there were no significant differences among the second to sixth periods (Fig. 2).
In all patients, including those with pathologic T3 prostate cancer, the mean operation time was 332±60, 231±50, 216±40, 203±35, 198±25, and 202±42 minutes, respectively, and it differed significantly between the first and second to sixth periods (p<0.001) (Fig. 1). Mean estimated blood loss was 1,013±936, 299±163, 487±402, 321±215, 318±241, and 286±172 ml, respectively, and it also differed significantly between the first and second to sixth periods (p<0.001) (Fig. 2). The first four patients (2%) in the series received blood transfusions.
The mean postoperative Gleason's score was 6.6 (range, 4-9), and the percentage of patients with a positive surgical margin was 13.2%. The positive surgical margin rate in the initial first year was 20.8%, and the rate declined with each period: 15.2%, 14.0%, 11.4%, and 9.5%, respectively (Fig. 3), but there was no significant difference among the periods (p=0.680). The mean duration of hospitalization was 5.1±3.0 days, and the mean duration of catheterization was 11.4±4.0 days.
Since Binder and Kramer first reported RALP in 2001 [1], RALP has come into wide use at a rapid rate as a result of its various technical advantages and short-term oncologic outcomes comparable with those of open radical prostatectomy and laparoscopic radical prostatectomy.
When a new surgical technique is introduced, it is compared with previous surgical techniques in terms of preoperative, intraoperative, and postoperative variables to assess its clinical and oncologic outcomes. Operation time, estimated blood loss, and positive surgical margin rate are important in the assessment of clinical and oncologic outcomes.
Initial RALP data showed relatively wide ranges in operation time: total operation time of 540 minutes (by Binder and Kramer [1]), robot installation time of 93 minutes and operation time of 222 minutes (by Pasticier et al [4]), and robot installation time of 0.95 hour and operation time of 4.8 hour (by Menon et al [5]). However, these operation times were reported in initial studies with sample sizes of fewer than 30 patients. As centers began performing more RALP procedures, larger studies demonstrated shorter operative times. Ahlering et al reported that, as the operator's experience accumulated, mean operation time was reduced to less than 4 hours after 12 cases of RALP with a mean operative time of 3.45 hours [6]. Thus, this report suggests that an operator without any experience with laparoscopic surgery can achieve a fast learning curve in performing RALP. Furthermore, Zorn et al analyzed 150 cases of RALP performed by skilled laparoscopic urologic surgeons [7]. In their report, the initial decrease in operative time to less than 4 hours was achieved after 120 cases and it was after 25 cases of RALP, without conversion to open radical prostatectomy and with no complications. Thus, Zorn et al concluded that inexperience in laparoscopy does not influence the learning curve. Mean operation time after the learning curve in other reports ranged from 141 to 250 minutes, and a gradual decline in operation time was also shown [8,9]. Our first case of RALP took 80 minutes for robot installation and 420 minutes for the operation [10]. However, as our number of experiences grew, we saw a major decline in operation time. Also, our initial decrease in operation time during the first 24 cases and gradual decrease after subsequent cases were similar with prior studies.
Low blood loss is often considered one of the main advantages of minimally invasive surgery. Pneumoperitoneum is thought to be a positive factor in decreasing the amount of blood loss. Ahlering et al compared open radical prostatectomy with RALP and stated that there were significant differences in blood loss between the two groups (418 ml vs. 103 ml) [11]. Furthermore, Tewari et al reported that blood loss was greater in open radical prostatectomy than in RALP (910 ml vs. 150 ml) [12]. According to data from larger studies with sample sizes of over 30 patients, mean blood loss ranged from 103 to 570 ml [11,13]. Jaffe et al analyzed 293 cases of RALP, subdividing the patients into 3 groups (before the first 12 cases, 13 to 188 cases, and after 189 cases) and showed that mean blood loss differed significantly among the 3 groups (242, 165, and 134 ml, respectively) [14]. In domestic studies, Ban et al divided patients into 2 groups (the first 15 cases and cases 16 to 50) and showed a significant difference in blood loss between the 2 groups (464 and 328 ml) [15]. As shown in our study, there was an abrupt decrease in estimated blood loss and a gradual decrease after the breakpoint.
In the present study, there was no significant difference in operation time or estimated blood loss between the patients with pathologic T2 prostate cancer and the total group of patients including those with pathologic T3 prostate cancer. Our results could be due to the fact that only 4 patients with pathologic T3 prostate cancer underwent RALP in the first and second periods, which showed steep learning curves. In addition, our results could be due to an inclusion of more patients with pathologic T3 prostate cancer after the surgeon achieved the learning curve.
Transfusion rates have been reported to range from 0% [5,11,12] to as high as 18% [16] in patients with RALP, but this rate has been shown to be significantly lower than with open radical prostatectomy. Tewari et al demonstrated this in comparing standard open radical prostatectomy with RALP [12]. They found a transfusion rate difference of 67% in their open group, compared with 0% in their robotic series.
There has not been enough long-term follow-up on oncologic outcomes of RALP. However, margin status can verify the surgical aspects of oncologic outcomes. Several studies comparing RALP with open radical prostatectomy and laparoscopic radical prostatectomy have demonstrated similar rates of positive surgical margin. After reviewing the current literature, Hegarty and Kaouk reported the positive margin rate of the open procedure, laparoscopic procedure, and RALP to be 13-21%, 16-26%, and 6-23%, respectively. Also, the positive margin rate is directly influenced by the stage of prostate cancer [17]. Wolfram et al reported a positive margin rate of 12.7% for pathologic T2 tumors and a positive margin rate of 42% for pathologic T3 tumors [9]. On the contrary, Jaffe et al reported rates of 23% for pathologic T2 tumors and 15% for pathologic T3 tumors [14]. We consider these differences to be because the positive margin rate is influenced directly not only by prostate cancer stage but also by operative techniques and nervesparing procedures. In addition, the surgeon's learning curve and each proportion of cancer stages per time period could have influenced the positive margin rate, because most studies are of initial experiences with RALP or short-term surgical outcomes. Our study was limited to pathologic T2 stage and showed an average positive margin rate of 13.2%, which is comparable with previously reported positive margin rates with the open procedure or laparoscopic procedure. Also, positive margin rates showed a tendency toward gradual decline as the surgeon's experiences accumulated. In our study, positive margin rates could not be statistically analyzed according to whether nerve-sparing procedures were performed because of the relatively small numbers of RALP performed without nerve-sparing procedures.
The results of the steep learning curve and positive margin rate in our study were not significantly different from studies of conventional open or laparoscopic procedures and are similar to the previous literature. We presume that the absence of tactile feedback, which has been the most troublesome disadvantage of RALP, will be overcome in the near future. Also, the continuous development of technologies in robotic systems should result in the surgeon's learning curve for RALP being achieved faster.
We intended to evaluate the clinical outcomes of RALP for pathologic T2 diseases and to investigate the learning curve. However, our study had several limitations. We assessed the learning curve based on a single surgeon's experience. Subjective variables such as surgeon's experiences in open and laparoscopic procedures and experiences in robotic surgery training abroad are included in this study. Thus, the results of our study cannot be applied to other surgeons in general. Furthermore, we evaluated the results of RALP on the basis of each period of time and assessed the learning curve. Differences in outcome may have existed according to the numbers of RALP procedures performed during each period of time. Better outcomes might have resulted if a relatively large number of RALP procedures were performed in a single period of time, thus enhancing the surgeon's proficiency.
Our study showed a gradual decrease in operative time and estimated blood loss during RALP with the da Vinci™ robot system. In addition, operation time and estimated blood loss had steep learning curves during the early 24 cases and then stabilized. Even though there was no significant difference in the positive surgical margin rate, a continuous decrease in the positive surgical margin rate was observed in our study. The short-term outcomes of our study verify the achievement of an excellent learning curve in RALP.
References
1. Binder J, Kramer W. Robotically-assisted laparoscopic radical prostatectomy. BJU Int. 2001; 87:408–410. PMID: 11251539.

2. Kong GS, Seong YK, Sung GT. Robotic-assisted radical prostatectomy using da Vinci™ surgical robotic system: initial Korean experience. Korean J Urol. 2005; 46:353–359.
3. Park SY, Ham WS, Choi YD, Rha KH. Robot-assisted laparoscopic radical prostatectomy: clinical experience of 200 cases. Korean J Urol. 2008; 49:215–220.

4. Pasticier G, Rietbergen JB, Guillonneau B, Fromont G, Menon M, Vallancien G. Robotically assisted laparoscopic radical prostatectomy: feasibility study in men. Eur Urol. 2001; 40:70–74. PMID: 11528179.

5. Menon M, Tewari A, Baize B, Guillonneau B, Vallancien G. Prospective comparison of radical retropubic prostatectomy and robot-assisted anatomic prostatectomy: the Vattikuti Urology Institute experience. Urology. 2002; 60:864–868. PMID: 12429317.

6. Ahlering TE, Skarecky D, Lee D, Clayman RV. Successful transfer of open surgical skills to a laparoscopic environment using a robotic interface: initial experience with laparoscopic radical prostatectomy. J Urol. 2003; 170:1738–1741. PMID: 14532766.

7. Zorn KC, Orvieto MA, Gong EM, Mikhail AA, Gofrit ON, Zagaja GP, et al. Robotic radical prostatectomy learning curve of a fellowship-trained laparoscopic surgeon. J Endourol. 2007; 21:441–447. PMID: 17451340.

8. Patel VR, Tully AS, Holmes R, Lindsay J. Robotic radical prostatectomy in the community setting--the learning curve and beyond: initial 200 cases. J Urol. 2005; 174:269–272. PMID: 15947662.

9. Wolfram M, Bräutigam R, Engl T, Bentas W, Heitkamp S, Ostwald M, et al. Robotic-assisted laparoscopic radical prostatectomy: the Frankfurt technique. World J Urol. 2003; 21:128–132. PMID: 12851781.

10. Lee YS, Han WK, Yang SC, Rha KH. Robot-assisted laparoscopic radical prostatectomy. Korean J Urol. 2006; 47:206–210.

11. Ahlering TE, Woo D, Eichel L, Lee DI, Edwards R, Skarecky DW. Robot-assisted versus open radical prostatectomy: a comparison of one surgeon's outcomes. Urology. 2004; 63:819–822. PMID: 15134953.

12. Tewari A, Srivasatava A, Menon M. A prospective comparison of radical retropubic and robot-assisted prostatectomy: experience in one institution. BJU Int. 2003; 92:205–210. PMID: 12887468.

13. Bentas W, Wolfram M, Jones J, Bräutigam R, Kramer W, Binder J. Robotic technology and the translation of open radical prostatectomy to laparoscopy: the early Frankfurt experience with robotic radical prostatectomy and one year follow-up. Eur Urol. 2003; 44:175–181. PMID: 12875935.

14. Jaffe J, Castellucci S, Cathelineau X, Harmon J, Rozet F, Barret E, et al. Robot-assisted laparoscopic prostatectomy: a single-institutions learning curve. Urology. 2009; 73:127–133. PMID: 18952261.

15. Ban JH, Ko YH, Kang SH, Park HS, Cheon J. Learning curve with robotic-assisted laparoscopic radical prostatectomy: a prospective study. Korean J Urol. 2009; 50:140–147.

16. Sim HG, Yip SK, Lau WK, Tan JK, Cheng CW. Early experience with robot-assisted laparoscopic radical prostatectomy. Asian J Surg. 2004; 27:321–325. PMID: 15564188.

17. Hegarty NJ, Kaouk JH. Radical prostatectomy: a comparison of open, laparoscopic and robot-assisted laparoscopic techniques. Can J Urol. 2006; 13(Suppl 1):56–61. PMID: 16526984.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download