Abstract
With the development of techniques for percutaneous access and equipment to disintegrate calculi, percutaneous nephroscopic surgery is currently used by many urologists and is the procedure of choice for the removal of large renal calculi and the management of diverticula, intrarenal strictures, and urothelial cancer. Although it is more invasive than shock wave lithotripsy and retrograde ureteroscopic surgery, percutaneous nephroscopic surgery has been successfully performed with high efficiency and low morbidity in difficult renal anatomies and patient conditions. These advantages of minimal invasiveness were rapidly perceived and applied to the management of ureteropelvic junction obstruction, calyceal diverticulum, infundibular stenosis, and urothelial cancer. The basic principle of endopyelotomy is a full-thickness incision of the narrow segment followed by prolonged stenting and drainage to allow regeneration of an adequate caliber ureter. The preferred technique for a calyceal diverticulum continues to be debated. Excellent long-term success has been reported with percutaneous, ureteroscopic, and laparoscopic techniques. Each approach is based on the location and size of the diverticulum. So far, percutaneous ablation of the calyceal diverticulum is the most established minimally invasive technique. Infundibular stenosis is an acquired condition usually associated with inflammation or stones. Reported series of percutaneously treated infundibular stenosis are few. In contrast with a calyceal diverticulum, infundibular stenosis is a more difficult entity to treat with only a 50-76% success rate by percutaneous techniques. Currently, percutaneous nephroscopic resection of transitional cell carcinoma in the renal calyx can be applied in indicated cases.
Go to : 
Percutaneous renal puncture was first described in 1955 by Goodwin and Casey, who placed a trocar directly into the collecting system [2]. Later, the Seldinger method of nephrostomy placement was adopted, in which a fine guide wire is placed into the collecting system through the core of the needle that was used to perform the initial renal puncture. A coaxial catheter can then be placed over this initial guide wire and the renal pelvis drained even if it was not dilated. The addition of a preformed pigtail to these nephrostomy catheters ensured that they could not be easily displaced from the pelvis. In 1976 Fernstrom and Johansson described a method of dilating such an antegrade nephrostomy by use of graded plastic dilators introduced coaxially down the track [3]. After a number of days, the tract was used for intrarenal manipulation utilizing Dormia baskets and other grasping tools. Currently, the percutaneous tract can be dilated by acute dilation with a balloon catheter. Percutaneous nephrostomy (PCN) is, and will continue to be, the cornerstone of every percutaneous procedure of the upper urinary tract.
Go to : 
The topographical position of the kidney depends on its embryological development. Classically, the pelvis lies opposite the lower border of the first lumbar vertebra on the right and slightly higher on the left.
Among numerous factors, enveloping fascia, vascular connections, and intra-abdominal pressure are probably the most important factors holding the kidney in position. Within the renal fascia, the surrounding fat allows a considerable amount of renal movement despite its apparent density, although the kidney is tethered by the short vessels rigidly anchored to their midline connections. Abdominal tone provided by the anterior abdominal wall may be the most important factor for renal stability. The position of the liver limits the cranial movement of the kidney on the right side. The proximity of the pancreas to the anterior aspect of the left kidney is said to be especially important in limiting the movement of the kidney. The suprarenal attachments and ligaments to the liver and duodenum probably do not play an important role in maintaining renal position [2].
Movement of the diaphragm in respiration causes the kidney to move downward in inspiration and upward in expiration. The amplitude of movement is very variable, but it is usually within 3-5 cm. Such movement is more pronounced in women than in men and in the right than in the left kidney. When the patient is in the prone position with bolsters under the chest and upper abdomen, the kidneys are further displaced in a cephalad direction.
Go to : 
The main renal artery divides into two main branches, the anterior and the posterior. The anterior division further subdivides into the four anterior segmental arteries, which supply the anterior and polar areas of the kidney. The posterior segmental artery supplies the rest of the posterior area of the kidney. In more than 50% of kidneys, the posterior segmental artery is located in the middle or upper half of the posterior renal surface, and it may be damaged with an excessively medial needle puncture of an upper calyx. The segmental arteries divide into the interlobar arteries after crossing the renal sinus and become the arcuate arteries at the corticomedullary junction. The interlobular arteries branch off the arcuate arteries at right angles. The Brodel line delineates an avascular plane between the anterior and the posterior blood supplies. By taking a posterolateral transparenchymal path, the needle traverses the area of the Brödel line, and damage to major blood vessels can be avoided. A direct posterior puncture that is too medial risks injury to the posterior segmental artery, which is the artery most commonly injured in endourologic procedures. A needle directed end-on to a posterior calyx passes transparenchymally, and the chance of significant bleeding is minimized.
Go to : 
Although Rupel and Brown first removed a renal calculus through an operatively established nephrostomy tract in 1941 [4], the first PNL via a nephrostomy tract created for the sole purpose of stone removal was performed in 1976 by Fernstrom and Johansson [2]. The introduction of this technique was further refined over the years. As operative technique and endoscopic equipment improved, PNL was performed with increasing efficacy and decreasing complications [5]. PNL has replaced open techniques in removing complex urinary calculi in most institutions. In Korea, the first PNL was performed in 1984 by Koh et al [6]. This operation has now spread to several other institutes in Korea [7-9].
The practice of PNL has changed over time and is continuing to evolve. Differing aspects of the procedure, such as the ideal dilating method, the type of nephrostomy tube used, and the technique of treating calyceal diverticula, have been debated. Even the need for a nephrostomy tube has been questioned.
I want to first introduce the usual method of performing percutaneous surgery, and each different method will be discussed later. An open-ended 5-6 Fr ipsilateral ureteral catheter or occlusion balloon catheter is passed, allowing the injection of contrast material to opacify and distend the collecting system. Once the ureteral catheter is inserted, the patient is placed in a prone position on a C-arm compatible table. The patient can also be placed in a lateral position and punctured under the guidance of ultrasonography. Bolsters are placed next to the patient and a sterile drape is applied to the C-arm, enabling its manipulation by the surgeon.
The radiation source is positioned under the patient to minimize scattered radiation exposure to the surgeon. The emission tube is shielded by an additional layer of material, which also reduces the scattered radiation to the operator.
It is very important to select the percutaneous tract. The preferred approach is by way of a posterior calyx because major vascular structures surrounding the renal pelvis can be avoided and the transparenchymal route stabilizes the catheter in an appropriate position. Approach through a short tract perpendicular to the convexity of the kidney causes minimal anatomical or functional damage if the tract is dilated by using a graded coaxial dilator. A puncture either too medial or too lateral will enter the renal pelvis directly. Direct puncture of the renal pelvis should be avoided because it carries a significant risk of injury to the posterior branch of the renal artery. Furthermore, the tract created from such a puncture provides no stability for the nephrostomy tube because it lacks parenchymal support. A computerized tomogram taken in a prone position could be helpful in a patient with anatomical abnormalities such as a horseshoe kidney. After opacification of the collecting system by injection of a contrast material through the ureteral catheter, a puncture is performed in mid-inspiration by use of the sheathed needle. The puncture tract should be straight to the aimed calyx to prevent a false tract during dilation. The position of the needle tip should be checked intermittently by rotating the C-arm. When the needle appears to be in a calyx, the stylet is removed while the sheath is a little bit advanced in position to the calyx, and the correct needle position is verified by aspiration of urine. At times, aspiration of urine might be delayed due to increased mucosity with injected contrast medium or not enough space between the stone and the calyx. Next, the guide wire is inserted and advanced with the sheath held immobile by the other hand. The guide wire is advanced until resistance is encountered, and its position is checked by the C-arm at this time. The puncture could be performed under the guidance of ultrasonography. It is easy to make a nephrostomy tract, but dilation of the tract should be performed under fluoroscopy.
If one has punctured into a calyx whose neck is filled by a stone, it may be difficult to pass the guide wire into the pelvis. In most of these situations, however, there is a narrow space between the stone and the calyx and one can try to manipulate the guide wire (sometimes a J-tipped guide wire) beyond the stone by using and in-and-out movement of the puncture needle or a preformed catheter such as a 'cobra' catheter. It is not recommended to dilate over the guide wire when the stiff portion of the guide wire has not passed to the calyx or pelvis, because of the great possibility of 'flipping' the wire out of the system during dilation and thereby losing the tract.
After the guide wire is well positioned, the needle is removed and a 1 cm incision is made at the wire site. The tract is dilated over the guide wire up to 26-30 Fr. Efficient dilation is dependent on maintaining the same track throughout the procedure, so that each dilator is following the same path into the kidney. The wire must be stiff enough to support the dilatation. Ideally, it reaches down the ureter into the bladder to avoid dislodgment during the use of the fascial dilators. When placement of the guide wire down the ureter is not feasible, positioning it in a calyx that is distant from the initial nephrostomy tract prevents its dislodgment during dilatation. Some urologists advocate the use of a second, safety guide wire in addition to the initial working guide wire. This safety wire is inserted adjacent to the working wire, its goal being to maintain access to the nephrostomy tract if the working wire is kinked or displaced. This safety wire is kept until the whole surgical procedure is finished.
A variety of techniques can be utilized to perform the tract dilatation. The most commonly used dilation techniques are the Amplatz dilator set or the 10 cm, 24-30 Fr dilating balloon catheter and sheath set. Balloon dilation catheters of the 9 Fr size can dilate a nephrostomy tract to a diameter of 24-30 Fr under pressure up to 16-20 atm in a one-step procedure. This may prove difficult or impossible if perirenal scar tissue from a previous surgery prevents complete expansion of the balloon over its entire length. Sequential plastic dilators allow stepwise dilation of the tract under fluoroscopic control; however, on withdrawal for insertion of the next larger dilator, compression of the tract is lost intermittently and bleeding occurs into the collecting system, sometimes hindering subsequent endoscopy. Coaxial metal dilators (each dilator slides over the next smaller one) allow stepwise tract dilation even in the presence of severe scarring with continuous nephrostomy tract compression for improved hemostasis. With any dilation technique, the last step is insertion of a working sheath, which may be either the 24-26 Fr metal working sheath of the nephroscope or a larger plastic sheath. A 28-30 Fr plastic working sheath is preferable to a metal nephroscope sheath in all cases in which extensive, prolonged instrumentation is anticipated (e.g., staghorn stones). Larger plastic sheaths not only provide better irrigation with lower intrapelvic pressures than do continuous-flow nephroscope sheaths but also allow easier extraction of large stone fragments. The stone can be fragmented with intracorporeal lithotriptors (e.g., holmium: YAG laser, the electrohydraulic lithotripter, ultrasonic lithotriptor, the pneumatic lithotriptor, and a combination ultrasonic-pneumatic device) and removed with various kinds of forceps and baskets.
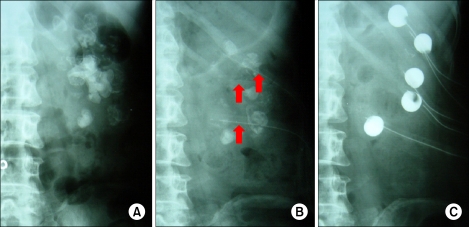
How many tracts are enough to remove all of the stone? The fewer the number of punctures, the better the overall renal functional outcome. But to achieve a better stone-free rate, one can make multiple accesses. I had a patient with a staghorn calculi in a solitary kidney who was treated with 8 percutaneous tracts to remove all of the muddy caliceal stones (Fig. 1). But the factors associated with significant blood loss are diabetes, multiple tracts, prolonged operative times, and the occurrence of intraoperative complications [10].
Percutaneous drainage of the pelvicaliceal system is routine after most endourologic approaches to the upper urinary tract. Some authors argue that there is no need for a drainage tube after certain percutaneous procedures [11]. Nevertheless, there seems to be concurrence in the literature regarding the need for postoperative drainage with a nephrostomy tube after percutaneous procedures. The desired function of the nephrostomy tube greatly influences the choice of which drainage method to adopt. The main function of a nephrostomy tube is the drainage of urine and possibly the tamponade of bleeding originating from the structures acutely expanded during dilatation. Kader and colleagues [12] reported that a small drainage catheter was associated with a shorter median length of hospital stay and a lesser analgesic requirement but no difference in the mean hemoglobin decrease compared with a large drainage catheter.
The success rates differ with the size, location, and composition of the stones; the collecting system anatomy; the endoscopic energy source; and the presence or absence of hydronephrosis [13].
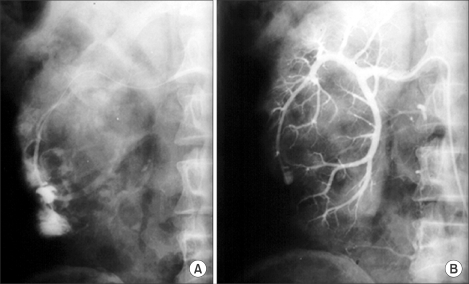
Bleeding is the most significant complication of PNL, with transfusion rates varying from less than 1% to 10%. Bleeding from an arteriovenous fistula or pseudoaneurysm requiring emergency embolization is seen in less than 0.5% of patients (Fig. 2) [14]. Most bleeding is venous in nature, and placement of a nephrostomy tube is usually adequate to control the bleeding. Clamping the nephrostomy tube for 10 minutes is helpful in tamponading any persistent bleeding [15]. Recently, there have been some reports of the use of fibrin glue to seal the nephrostomy tract following PCNL (tubeless) [16,17].
PNL can lead to some absorption of irrigation fluid; therefore, the use of physiologic irrigating solutions is essential. The amount of absorbed fluid depends mostly on the irrigant pressure and the length of the procedure. Intraoperative administration of diuretics (e.g., mannitol 12.5 g) is advisable and also has proved effective in preventing intrarenal reflux [18].
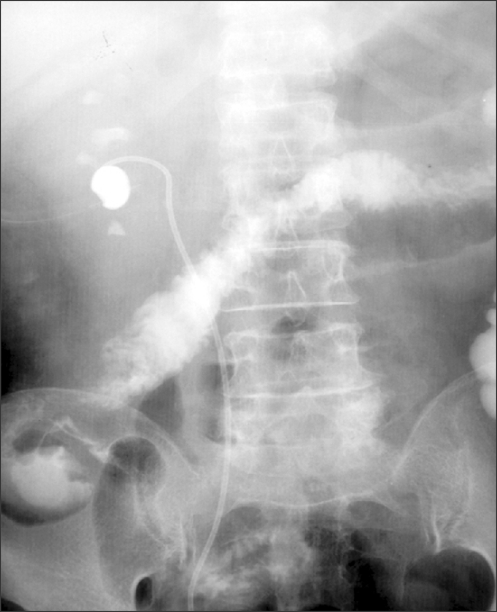
When a supracostal puncture is performed, extravasation of the irrigant may occur into the pleural cavity. The use of a working sheath tends to minimize extravasation into the pleura because intrarenal pressure is low. The chest should be examined at the end of PNL procedures in which a supracostal puncture is used. When supracostal puncture is performed, the risk of a pneumothorax or pleural effusion requiring drainage is 4% to 12% [18,19]. Punctures above the 11th rib result in a tremendously higher intrathoracic complication rate (34.6%) compared with the supra 12th rib access (1.4%) [20]. These facts corroborate the strategy of avoiding this high approach as much as possible. If the clinical findings suggest either of these complications, placement of a chest tube is mandatory. Immediate aspiration is performed, and the tube is removed within 24 hours. If the hemothorax is extensive, a large chest tube is advisable. Pardalidis and Smith suggested that in the case of nephrostomy access between the 11th and 12th rib, approximately 10% of patients present with fluid accumulation within the pleural space [21]. Colonic injury is an unusual complication often diagnosed on a postoperative nephrostogram (Fig. 3). Colonic injury tends to occur in severe lean or retrorenal colon patients, so one should be careful not to injure the colon during puncture and tract dilation in these patients. Typically, the injury is retroperitoneal; thus, signs and symptoms of peritonitis are infrequent. If the perforation is extraperitoneal, management may be expectant, with placement of a ureteral catheter or double-J stent to decompress the collecting system and withdrawal of the nephrostomy tube from an intrarenal position to an intracolonic position to serve as a colostomy tube. The colostomy tube is left in place for a minimum of 7 days and is removed after a nephrostogram or a retrograde pyelogram showing no communication between the colon and the kidney [22,23]. Exploration is recommended if the colon injury is found late.
The effect of PNL on short-term differential renal function was examined with nuclear renography by Chatham et al [24]. 99m-Tc-mercaptoacetyl triglycine (MAG3) nuclear scans were performed preoperatively and postoperatively in 19 PNL patients. Nuclear renography at a median of 22 days revealed stable differential function in the treated kidney. Renal function was previously assessed in anatrophic nephrolithotomy patients with MAG3 and a decrease from 42.0% preoperatively to 37.6% postoperatively was noted [25]. Liou and Streem assessed long-term renal function in patients with solitary kidneys after shockwave lithotripsy (SWL), PNL, or combined PNL/SWL therapy [26]. With the use of serum creatinine and calculated GFR, follow-up renal function revealed no statistically significant change in any of the chosen therapeutic modalities. Although no significant differences in postoperative renal function were found among the different therapy options, the PNL and combined therapy group had an average post-procedural increase in Cr by 0.5 mg/dl, as compared with a 0.1-mg/dl decrease in the SWL only group.
Although PNL was not introduced until the 1980s, the role of PNL is firmly entrenched. Kerbl et al noted that the number of percutaneous stone procedures had steadily increased from 2068 cases to 2678 over the time period from 1988 to 2000 [27]. Not surprisingly, the number of percutaneous procedures performed for stone burden greater than 2 cm rose by 123%. These data confirm the recommendation of the NIH Consensus Conference for primary percutaneous therapy for larger stone burdens. PNL continues to play an important role in treating lower pole calculi. Although many lower pole stones are treated initially with SWL or even ureteroscopy, the Lower Pole Study Group revealed a clear advantage of PNL in stones over 1.0 cm [28]. The role of PNL as the primary therapy for lower pole calculi may accordingly increase. Although PNL is safe and effective, future studies may further refine the technique of PNL, help to minimize adverse effects, and thereby help to deliver better patient care.
Go to : 
Endourologic management of ureteropelvic junction (UPJ) obstruction was introduced by Whitfield and Wickham in 1983 as a 'percutaneous pyelolysis' and was popularized shortly thereafter by Smith, who coined the term 'endopyelotomy' [29]. Despite various nuances in the name of the procedure and in the technique performed, the basic concept is constant and involves a full-thickness incision through the obstructing proximal ureter from the ureteral lumen out to the peripelvic and periureteral fat. The incision is stented and left to heal based on the early work of Davis, who used an 'intubated ureterotomy' in the course of an open operative procedure for UPJ obstruction [30]. Smith introduced endopyelotomy with a live surgery workshop to Korean doctors in 1987. After that, endopyelotomy started to be reported in papers from Korea [31,32].
Contraindications to a percutaneous endopyelotomy are similar to the contraindications to any endourologic approach and include a long segment (>2 cm) of obstruction, active infection, or untreated coagulopathy. The impact of crossing vessels is controversial [33-36].
Compared with the retrograde techniques of endopyelotomy (incision with a cold knife, Acucise catheter, Greenwald electrode, or laser), the antegrade technique offers the advantage of an incision under direct vision. The incision must be extended into the perirenal fat and into the healthy ureter. Although several clinicians suggest that the incision should always be made laterally, in fact, the ureter may be inserted into the renal pelvis on the anterior or posterior wall. In such cases, the incision should instead marsupialize the proximal ureter into the renal pelvis such that an anterior or posterior incision may be required [30]. Percutaneous endopyeloplasty, horizontal percutaneous suturing of a conventional longitudinal endopyelotomy incision, was recently developed with good clinical results. The technical simplicity and shorter operative time are advantages compared with laparoscopic pyeloplasty [37,38].
Once the incision is complete, stenting is accomplished. A 14/7 Fr endopyelotomy stent can be used and is passed in an antegrade fashion with the large diameter end of the stent positioned across the UPJ. There was a trend for better results with the use of a 14/7 Fr stent in patients with secondary stricture, although the difference in success rates between the 6 Fr and the 14/7 Fr stent was not significant statistically [39].
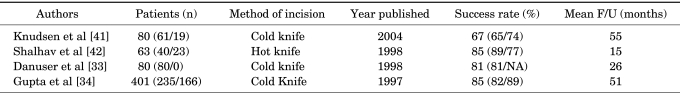
The immediate and long-term results of percutaneous endopyelotomy are well established. Clearly, percutaneous endopyelotomy compares favorably with open operative pyeloplasty in terms of postoperative pain, the length of hospital stay, and the return to prehospitalization activities [30,40]. Currently, success rates approaching 85% to 90% are reported at experienced centers (Table 1). Little difference in outcome is noted between primary and secondary UPJ obstruction and no difference in methods of incision.
Laparoscopic pyeloplasty has recently been reported, with success rates in excess of 95% [43]. Moreover, laparoscopy and robot-assisted surgery can be applied in patients with severe hydronephrosis requiring pelvic reduction and in patients with crossing vessels that may require ureteral-vascular transposition. However, the steep learning curve in laparoscopic surgery or the economical condition may limit laparoscopic or robot-assisted pyeloplasty to select centers proficient in reconstructive laparoscopy or robotic surgery.
Go to : 
A calyceal diverticulum is a smooth-walled, nonsecretory cavity in the renal parenchyma that is lined with transitional cell epithelium. It receives urine by passive retrograde filling from the adjacent collecting system, usually through a narrow forniceal channel or infundibulum. Calyceal diverticula are believed to be congenital in origin, likely from failed degeneration of small ureteral buds. They are typically less than 1 cm in diameter with no predilection for sex or kidney side. Uncomplicated, asymptomatic calyceal diverticula may be managed conservatively without routine follow-up imaging. However, because of their cystic, urine-containing nature, they are frequently associated with stone formation and infection and become symptomatic in up to one third of patients [44].
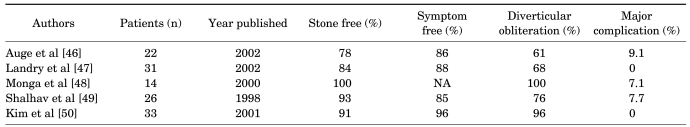
Treatment of a calyceal diverticulum containing a stone has evolved from open surgical excision to SWL, to percutaneous and ureteroscopic ablative techniques. The preferred technique continues to be debated. Percutaneous management of the calyceal diverticulum is challenging because the cavity is often small, making localization for direct access difficult. The first Korean paper was reported in 1994 and the percutaneous process was mentioned [45]. Cystoscopy and ureteral balloon catheter placement is performed in the renal pelvis. A balloon catheter is helpful to opacify the diverticulum with injection of contrast through the ureteral catheter to guide the percutaneous access, especially when the neck of the diverticulum is narrow. Direct puncture of the diverticulum is then made under fluoroscopic guidance, and a guide wire is coiled within it. Ideally, a polytetrafluoroethylene-coated or hydrophilic safety wire is placed through the diverticular neck into the renal pelvis, but it is usually coiled in the diverticular cavity because the neck cannot be cannulated. Canales and Monga advocate dilation of the tract into the diverticulum, although not through the diverticular neck because the goal of the procedure is to ablate the cavity and the connection to the collecting system [44]. Dilation of the diverticular infundibulum could be viewed as counterproductive. Usually, one can find the diverticular neck with the nephroscope during the injection of indigo carmine through the ureteral balloon catheter. Auge et al described an alternative approach if guide wire passage into the main collecting system was unsuccessful after several attempts [46]. Once inside the cavity, they advance an 18 gauge percutaneous access needle directly through the inner or medial diverticulum wall into the renal collecting system and subsequently dilate to 30 Fr with a dilating balloon, creating a large 'neoinfundibulotomy' tract. This maneuver prevents the safety wire from being inadvertently withdrawn. With this technique, the connection between the diverticulum and the collecting system is enlarged rather than ablated. But in my experience, I never find a diverticulum without a diverticular neck. The urothelium of a calyceal diverticulum is usually fulgurated with electrocautery or a holmium laser if greater than 4 cm in diameter. If electrocautery is utilized, the safety wire should be insulated with an open-ended catheter to prevent inadvertent transmission of the current down the ureter. The nephrostomy catheter is placed through the calyceal diverticulum and neoinfundibulotomy and secured in the renal pelvis. There are controversies about the duration of a nephrostomy catheter. But it tends to be shorter because there is no difference in success rates according to nephrostomy catheter duration [44,46,47]. The results of percutaneous management of calyceal diverticula from the literature are presented in Table 2. In the cases reviewed, stone-free rates and symptom-free rates for percutaneous management are consistently 80% or greater. Minor complications during percutaneous ablation and calculus removal include hemorrhage, pneumothorax, persistent urinary extravasation, and mild extravasation of irrigant. Major complications include renal pelvis perforation with urinoma formation, pneumothorax or hemothorax requiring tube thoracostomy, and massive hemorrhage requiring balloon tamponade. As the table demonstrates, major complications are relatively uncommon.
Limitations exist primarily for an anteriorly located diverticulum. In this situation, if the diverticulum is in a superior anterior calyx, the ureteroscopic approach is recommended, whereas if the diverticulum is in a middle or lower anterior calyx, a laparoscopic approach is recommended [44]. The ureteroscopic approach may be an appropriate initial treatment option for patients with a small stone burden (<1.5 cm) or patients with comorbidities who are poor candidates for PNL.
Go to : 
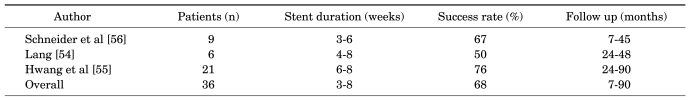
Infundibular stenosis and hydrocalyx are usually an acquired condition associated with inflammation, renal tuberculosis, obstructive calculus, or prior renal surgery [18,51]. The hydrocalyx should be differentiated from a calyceal diverticulum because the treatments are different. At times, this distinction can be made only by a nephroscopy because the presence (hydrocalyx) or absence (calyceal diverticulum) of a renal papilla is diagnostic. The infundibular narrowing can be resolved in several ways. The least difficult approach is to dilate the infundibulum to 8 mm with an 8 mm ureteral dilating balloon passed over the working guide wire. Alternatively, the infundibulum can be cut under endoscopic control with a cold knife through a direct vision ureterotome. When the guide wire cannot be passed through the stricture, a round tipped rigid ureteroscope can be pushed in an antegrade fashion to traverse the stricture with injection of indigo carmine through the retrograde ureteral catheter (Fig. 4). According to anatomic studies by Sampaio, the incision should be made along the less vascular superior and inferior aspects of the middle calyceal infundibulum or the medial and lateral aspects of the upper calyceal infundibulum [52]. Reported series of endourologically treated infundibular stenosis are few. The first Korean case report of endoscopic infundibulotomy was published by Han et al in 1991 [53]. Also, Lang reported a 50% success rate in 6 patients with infundibular stenosis and caliceal diverticula containing stones [54]. Hwang and Park reported an 80% success rate in 10 patients with tuberculous infundibular strictures who had undergone a cold knife incision; follow-up was greater than 1 year [51]. Hwang et al reported long-term (more than 2 years follow-up) results with a success rate of 76% in 21 patients and better results in the strictures with stones than in the strictures with tuberculosis [55]. It appears that in contrast to the calyceal diverticulum, in which a successful outcome is obtainable in nearly 90% of cases, the infundibular stenosis is a more difficult entity to treat endourologically, with only a 50% to 76% success rate (Table 3).
Go to : 
Nephroureterectomy with bladder cuff excision has been considered the "gold standard" for upper-tract transitional cell carcinoma (TCC). In upper-tract TCC, endoscopic surgery could be indicated in those patients with a solitary kidney, bilateral disease, high surgical risk, or chronic renal insufficiency and incidental discovery of the tumor [57,58].
The smooth muscle covering the ureter and renal pelvis is much thinner than the bladder wall. Therefore, tumors in the ureter and renal pelvis can penetrate the wall earlier. Indeed, approximately 70% of upper-tract TCCs were of moderate or high grade, and more than half had some degree of invasion [59].
Endoscopic surgery (ureteroscopy and percutaneous nephroscopy) has been used in the treatment of upper-tract tumors since the 1980s [60-62]. Endoscopic surgery may be considered for those patients with low-grade, low-stage cancer malignancies under an intensive surveillance program. Percutaneous approaches show recurrence rates and disease-specific survival in the patients with low-grade tumors (Grades 1-2) of 26% to 28% and 96% to 100%, respectively [57]. Lee et al compared patients undergoing nephroureterectomy versus percutaneous resection and noted no statistically significant differences in survival for either treatment group (Grades 1, 2, and 3) [63]. Tumor grade was the most important prognostic indicator for pathologic stage, recurrence rates, and cancer-related deaths.
The PCN tract is made to the calyx under fluoroscopy. To approach the renal pelvis and upper ureter, a PCN tract through the upper-pole or middle posterior calyx is better to enable good vision. The tract is dilated up to 30 F to ensure a free flow of irrigation fluid at low pressure. The fresh tumor tissue is removed with cold-cup forceps and the whole tumorous area is fulgurated with electrocautery or laser. A 24 Fr nephrostomy tube is placed for a second-look nephroscopy 3 to 7 days later.
The advantages of the percutaneous approach are the use of larger instruments, better vision, complete resection of large tumors, deeper biopsies, and better staging [57,64]. Also, the percutaneous tract makes it easy to do a second look procedure and adjuvant topical therapy. Potential disadvantages include the increased morbidity and the theoretical concern over tract seeding. However, percutaneous tract seeding is rare in patients with upper-tract TCC.
Typically, a second look procedure is performed within several days of the initial procedure. Any remaining tumor is treated. Intracavitary adjuvant therapy (BCG or mitomycin) can be administered 2 weeks after the resection, assuming a nephrostogram is normal.
The most common agents used for topical adjuvant therapy are BCG, mitomycin, and thiotepa. Generally, intracavitary placement of these substances is well tolerated and the agents can be delivered percutaneously or in a retrograde fashion following ureteroscopy [65]. Six weekly courses of intracavitary BCG is recommended, and 2 weeks after completion of the full BCG course, third-look nephroscopy is recommended.
Follow-up of the patients consists of urine cytology study at 3 months, intravenous urography at 6 months, cystoscopy at 3 and 9 months, and flexible ureteropyeloscopy and computed tomography scan at 1 year [64].
Go to : 
Notes
Most of this article was previously published in Recent Advances in Endourology [1]. It is presented here under the permission of JSEE.
Go to : 
References
1. Hwang TK, Seo SI. Baba S, Ono Y, editors. Percutaneous access for urological disease. Recent advances in endourology. 2006. 8th ed. Japan: Springer;p. 25–39.

2. Gupta M, Ost MC, Shah JB, McDougall EM, Smith A. Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peter CA, editors. Percutaneous management of the upper urinary tract. Campbell-Walsh urology. 2007. 9th ed. Philadelphia: Saunders;p. 1526–1563.
3. Fernström I, Johansson B. Percutaneous pyelolithotomy. A new extraction technique. Scand J Urol Nephrol. 1976; 10:257–259. PMID: 1006190.
4. Rupel E, Brown R. Nephroscopy with removal of stone following nephrostomy for obstructive calculous anuria. J Urol. 1941; 46:177–182.

5. Lingeman JE, Newmark JR, Wong MY. Smith AD, editor. Classification and management of staghorn calculi. Controversies in endourology. 1995. Philadelphia: Saunders;p. 136–144.
6. Koh SK, Cho JP, Yoon DK, Cha IH. Percutaneous nephrolithotripsy. Korean J Urol. 1984; 25:739–745.
7. Lee MS, Chung BH. Early experience of percutaneous nephrolithotomy. Korean J Urol. 1986; 27:417–423.
8. Park CH, Lee SC. Percutaneous nephrolithotomy: clinical experience of 54 cases. Korean J Urol. 1986; 27:630–636.
9. Lee JH, Lee YJ, Hwang TK, Park YH. Percutaneous nephrolithotomy: 57 cases. Korean J Urol. 1989; 30:41–47.
10. Kukreja R, Desai M, Patel S, Bapat S, Desai M. Factors affecting blood loss during percutaneous nephrolithotomy: prospective study. J Endourol. 2004; 18:715–722. PMID: 15659890.
11. Goh M, Wolf JS Jr. Almost totally tubeless percutaneous nephrolithotomy: further evolution of the technique. J Endourol. 1999; 13:177–180. PMID: 10360497.

12. Kader AK, Finelli A, Honey RJ. Nephroureterostomy-drained percutaneous nephrolithotomy: modification combining safety with decreased morbidity. J Endourol. 2004; 18:29–32. PMID: 15006049.

13. Deane LA, Clayman RV. Advances in percutaneous nephrostolithotomy. Urol Clin North Am. 2007; 34:383–395. PMID: 17678988.

14. Kessaris DN, Bellman GC, Pardalidis NP, Smith AG. Management of hemorrhage after percutaneous renal surgery. J Urol. 1995; 153:604–608. PMID: 7861493.

15. Carson CC. Complications of percutaneous stone extraction: prevention and treatment. Semin Urol. 1986; 4:161–169. PMID: 3749655.
16. Mikhail AA, Kaptein JS, Bellman GC. Use of fibrin glue in percutaneous nephrolithotomy. Urology. 2003; 61:910–914. PMID: 12736002.

17. Shah HN, Kausik V, Hedge S, Shah JN, Bansal MB. Initial experience with hemostatic fibrin glue as adjuvant during tubeless percutaneous nephrolithotomy. J Endourol. 2006; 20:194–198. PMID: 16548728.

18. McDougall EM, Liatsikos EN, Dinlenc CZ, Smith AD. Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. Percutaneous approaches to the upper urinary tract. Campbell's urology. 2002. 8th ed. Philadelphia: Saunders;p. 3320–3360.
19. Golijanin D, Katz R, Verstandig A, Sasson T, Landau EH, Meretyk S. The supracostal percutaneous nephrostomy for treatment of staghorn and complex kidney stones. J Endourol. 1998; 12:403–405. PMID: 9847059.

20. Munver R, Delvecchio FC, Newman GE, Preminger GM. Critical analysis of supracostal access for percutaneous renal surgery. J Urol. 2001; 166:1242–1246. PMID: 11547050.

21. Pardalidis N, Smith AD. Smith AD, editor. Complications of stone treatment. Controversies in endourology. 1995. Philadelphia: WB Saunders;p. 179–185.
22. Wolf JS Jr. Management of intraoperatively diagnosed colonic injury during percutaneous nephrostolithotomy. Tech Urol. 1998; 4:160–164. PMID: 9800900.
23. Lingeman JE, Matlaga BR, Evan AP. Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Surgical management of upper urinary tract calculi. Campbell-Walsh urology. 2007. Philadelphia: Saunders;p. 1431–1507.
24. Chatham JR, Dykes TE, Kennon WG, Schwartz BF. Effect of percutaneous nephrolithotomy on differential renal function as measured by mercaptoacetyl triglycine nuclear renography. Urology. 2002; 59:522–525. PMID: 11927303.

25. Morey AF, Nitahara KS, McAninch JW. Modified anatrophic nephrolithotomy for management of staghorn calculi: Is renal function preserved? J Urol. 1999; 162:670–673. PMID: 10458338.

26. Liou LS, Streem SB. Long-term renal functional effects of shock wave lithotripsy, percutaneous nephrolithotomy and combination therapy: a comparative study of patients with solitary kidney. J Urol. 2001; 166:36. PMID: 11435817.

27. Kerbl K, Rehman J, Landman J, Lee D, Sundaram C, Clayman RV. Current management of urolithiasis: progress or regress? J Endourol. 2002; 16:281–288. PMID: 12184077.

28. Albala DM, Assimos DG, Clayman RV, Denstedt JD, Grasso M, Gutierrez-Aceves J, et al. Lower pole I: a prospective randomized trial of extracorporeal shock wave lithotripsy and percutaneous nephrostolithotomy for lower pole nephrolithiasis-initial results. J Urol. 2001; 166:2072–2080. PMID: 11696709.

29. Badlani G, Eshghi M, Smith AD. Percutaneous surgery for ureteropelvic junction obstruction (endopyelotomy): technique and early results. J Urol. 1986; 135:26–28. PMID: 3941462.

31. Lee MS, Yang SC, Choi YD, Chung BH. Endoscopic surgery for ureteropelvic junction obstruction. Korean J Urol. 1988; 29:83–88.
32. Kim DY, Kwon SH, Chung SK, Kim BW, Park YK. Endopyelotomy as a treatment for ureteropelvic junction obstruction - 3 cases. Korean J Urol. 1988; 29:434–440.
33. Danuser H, Ackermann DK, Böhlen D, Studer UE. Endopyelotomy for primary ureteropelvic junction obstruction: risk factors determine the success rate. J Urol. 1998; 159:56–61. PMID: 9400436.

34. Gupta M, Tuncay OL, Smith AD. Open surgical exploration after failed endopyelotomy: a 12-year perspective. J Urol. 1997; 157:1613–1618. PMID: 9112488.

35. Sampaio FJ. Vascular anatomy at the ureteropelvic junction. Urol Clin North Am. 1998; 25:251–258. PMID: 9633579.

36. Van Cangh PJ, Wilmart JF, Opsomer RJ, Abi-Aad A, Wese FX, Lorge F. Long-term results and late recurrence after endoureteropyelotomy: a critical analysis of prognostic factors. J Urol. 1994; 151:934–937. PMID: 8126829.

37. Gill IS, Desai MM, Kaouk JH, Wani K, Desai MR. Percutaneous endopyeloplasty: description of new technique. J Urol. 2002; 168:2097–2102. PMID: 12394717.

38. Desai MM, Desai MR, Gill IS. Endopyeloplasty versus endopyelotomy versus laparoscopic pyeloplasty for primary ureteropelvic junction. Urology. 2004; 64:16–21. PMID: 15245924.
39. Hwang TK, Yoon JY, Ahn JH, Park YH. Percutaneous endoscopic management of upper ureteral stricture size of stent. J Urol. 1996; 155:882–884. PMID: 8583598.

40. Brooks JD, Kavoussi LR, Preminger GM, Schuessler WW, Moore RG. Comparison of open and endourologic approaches to the obstructed ureteropelvic junction. Urology. 1995; 46:791–795. PMID: 7502417.

41. Knudsen BE, Cook AJ, Watterson CD, Beiko DT, Nott L, Razvi H, et al. Percutaneous antegrade endopyelotomy: long-term results from one institution. Urology. 2004; 63:230–234. PMID: 14972459.

42. Shalhav AL, Giusti G, Elbahnasy AM, Hoenig DM, McDougall EM, Smith DS, et al. Adult endopyelotomy: impact of etiology and antegrade versus retrograde approach on outcome. J Urol. 1998; 160:685–689. PMID: 9720521.

43. Ost MC, Kaye JD, Guttman MJ, Lee BR, Smith AD. Laparoscopic pyeloplasty versus antegrade endopyelotomy: comparison in 100 patients and a new algorithm for the minimally invasive treatment of ureteropelvic junction obstruction. Urology. 2005; 66(5 Suppl):47–51. PMID: 16194707.

44. Canales B, Monga M. Surgical management of the calyceal diverticulum. Curr Opin Urol. 2003; 13:255–260. PMID: 12692451.

45. Kang JO, Lee DH, Hwang TK. Endourologic management of caliceal diverticula containing calculi. Korean J Urol. 1994; 35:543–547.
46. Auge BK, Munver R, Kourambas J, Newman GE, Wu NZ, Preminger GM. Neoinfundibulotomy for the management of symptomatic caliceal diverticula. J Urol. 2002; 167:1616–1620. PMID: 11912375.

47. Landry JL, Colombel M, Rouviere O, Lezrek M, Gelet A, Dubernard JM, et al. Long term results of percutaneous treatment of caliceal diverticular calculi. Eur Urol. 2002; 41:474–477. PMID: 12074821.

48. Monga M, Smith R, Ferral H, Thomas R. Percutaneous ablation of caliceal diverticulum: long-term followup. J Urol. 2000; 163:28–32. PMID: 10604307.

49. Shalhav AL, Soble JJ, Nakada SY, Wolf JS Jr, McClennan BL, Clayman RV. Long-term outcome of caliceal diverticula following percutaneous endosurgical management. J Urol. 1998; 160:1635–1639. PMID: 9783921.

50. Kim JW, Seo SI, Hwang TK. Percutaneous treatment of caliceal diverticular stone. Korean J Urol. 2001; 42:180–184.
51. Hwang TK, Park YH. Endoscopic infundibulotomy in tuberculous renal infundibular stricture. J Urol. 1994; 151:852–854. PMID: 8126808.

52. Sampaio FJ. Smith AD, Badlani GH, Bagley DH, Clayman RV, Jordan GH, Kavoussi LR, editors. Surgical anatomy of the kidney. Smith's textbook of endourology. 1996. St Louis: Quality Medical;p. 153–184.
53. Han CH, Yang DK, Hwang TK, Park YH. Early experience of endoscopic infundibulotomy. Korean J Urol. 1991; 32:807–810.
54. Lang EK. Percutaneous infundibuloplasty: management of calyceal diverticula and infundibular stenosis. Radiology. 1991; 181:871–877. PMID: 1947113.

55. Hwang TK, Seo SI, Kim JC, Yoon JY, Park YH, Yoon MS. Long-term results of percutaneous endourologic management of renal infundibular stricture. J Endourol. 1999; 13:495–498. PMID: 10569522.

56. Schneider AW, Conrad S, Busch R, Otto U. The cold-knife technique for endourological management of stenoses in the upper urinary tract. J Urol. 1991; 146:961–965. PMID: 1895451.

57. Soderdahl DW, Fabrizio MD, Rahman NU, Jarrett TW, Bagley DH. Endoscopic treatment of upper tract transitional cell carcinoma. Urol Oncol. 2005; 23:114–122. PMID: 15869996.

58. Box GN, Lehman DS, Landman J, Clayman RV. Minimally invasive management of upper tract malignancies: renal cell and transitional cell carcinoma. Urol Clin North Am. 2008; 35:365–383. PMID: 18761193.

59. Stewart GD, Bariol SV, Grigor KM, Tolley DA, McNeill SA. A comparison of the pathology of transitional cell carcinoma of the bladder and upper urinary tract. BJU Int. 2005; 95:791–793. PMID: 15794784.

60. Tasca A, Zattoni F. The case for a percutaneous approach to transitional cell carcinoma of the renal pelvis. J Urol. 1990; 143:902–904. PMID: 2329603.

61. Woodhouse CR, Kellett MJ, Bloom HJ. Percutaneous renal surgery and local radiotherapy in the management of renal pelvic transitional cell carcinoma. Br J Urol. 1986; 58:245–249. PMID: 3719242.

62. Orihuela E, Smith AD. Percutaneous treatment of transitional cell carcinoma of the upper urinary tract. Urol Clin North Am. 1988; 15:425–431. PMID: 3407033.

63. Lee BR, Jabbour ME, Marshall FF, Smith AD, Jarrett TW. 13-year survival comparison of percutaneous and open nephroureterectomy approaches for management of transitional cell carcinoma of renal collecting system: equivalent outcomes. J Endourol. 1999; 13:289–294. PMID: 10405908.

64. Jabbour ME, Smith AD. Primary percutaneous approach to upper urinary tract transitional cell carcinoma. Urol Clin North Am. 2000; 27:739–750. PMID: 11098771.

65. Lamm DL, Blumenstein BA, Crissman JD, Montie JE, Gottesman JE, Lowe BA, et al. Maintenance bacillus calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000; 163:1124–1129. PMID: 10737480.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download