Abstract
Purpose
A retroaortic left renal vein (RLRV) is located between the aorta and the vertebra and drains into the inferior vena cava. Urological symptoms can be caused by increased pressure in the renal vein. To evaluate the clinical importance of RLRV, we reviewed patients' medical records and radiologic findings.
Materials and Methods
Nine patients who were studied with multidetector computed tomography at our institution from January 2003 to December 2009 had urologic symptoms with RLRV. We retrospectively reviewed these patients' medical records and analyzed their clinical characteristics.
Results
The patients' mean age was 46.0±20.1 years (range, 17-65 years) and the male to female ratio was 5 to 4. The urologic symptoms of the initial diagnosis were various (hematuria: 5 of the 9 patients; left flank pain: 4 of the 9 patients; inguinal pain: 1 of the 5 male patients; and gross hematuria: 1 of the 9 patients). The distribution among the type I, II, III, and IV of RLRV was 6, 2, 1, and 0 patients, respectively. The concomitant diseases were ureteropelvic junction obstruction (UPJO; 2 of the 9 patients) and varicocele (2 of the 5 male patients). One patient with UPJO underwent pyeloplasty and the other patient with UPJO underwent nephrectomy due to a nonfunctional atrophied kidney. The microscopic hematuria was not resolved with conservative management for long-term follow-up.
A left renal vein passing behind the abdominal aorta is termed a retroaortic left renal vein (RLRV), and this anomaly is a relatively uncommon condition. Recent advances in computed tomography and magnetic resonance imaging techniques make it possible to visualize the vascular structures in detail. Additionally, the congenital anomalies of the inferior vena cava (IVC) and its tributaries have become more frequently encountered in asymptomatic patients. RLRV anomalies, although usually overlooked, are not rare [1,2]. However, only a few cases showing clinical symptoms with this anomaly have been reported [3]. It is also of surgical importance when a left renal surgery is considered. Failure to recognize these anomalies may lead to severe hemorrhage and severe renal damage [4]. Compression of the RLRV between the aorta and the vertebra is known to be the cause of urological problems such as hematuria, varicocele, and ureteropelvic junction obstruction (UPJO) [5].
We retrospectively evaluated the type, frequency, clinical significance, management, and long-term follow-up of RLRV in 9 patients who were examined with multidetector computed tomography (MDCT) owing to urological problems.
Twelve patients with urological problems, such as hematuria, flank and abdominal pain, varicocele, and UPJO, were studied with MDCT at our institution from January 2003 to December 2009. Full follow-up information was available for 9 of the 12 patients. We retrospectively reviewed these patients' medical records and analyzed their clinical characteristics including sex, age, type of RLRV, urologic symptoms, concomitant disease, treatment, and follow-up results (Table 1).
The mean age and follow-up duration of the 9 patients were 46.0±20.1 years (range, 17-65 years) and 32.8±20.1 months (range, 15-69 months), respectively, and the male to female ratio was 5 to 4. The urologic symptoms of the initial diagnosis were various (microscopic hematuria: 5 of the 9 patients; left flank pain: 4 of the 9 patients; inguinal pain: 1 of the 5 male patients; and gross hematuria: 1 of the 9 patients), and the concomitant diseases were UPJO in 2 of the 9 patients and varicocele in 2 of the 5 male patients. The number of patients with type I, II, III, and IV of RLRV were 6, 2, 1, and 0 patients, respectively. In type II, all patients had UPJO and 1 of the patients had varicocele. One of these patients underwent laparoscopic pyeloplasty, and the other patient underwent laparoscopic nephrectomy owing to a nonfunctional kidney with varicocelectomy.
Symptoms in the five patients with microscopic hematuria continued, whereas 1 patient with gross hematuria had resolved gross hematuria after nephrectomy. The two patients with UPJO who underwent pyeloplasty and nephrectomy were treated successfully, and in the other 2 patients, the pain spontaneously resolved. The symptoms in the one patient with varicocele also spontaneously improved (Table 1). However, the microscopic hematuria was not resolved with conservative management for long-term follow-up.
The development of the renal veins is a part of the complex developmental process of the IVC. The process starts from the fourth week of conception and ends at about the eighth week. The IVC is formed from a vast network of three pairs of parallel veins in communication. The posterior cardinal veins, the subcardinal veins, and the supracardinal veins are in the order of appearance [8,9]. During the development of the IVC, there are anastomotic communications between the subcardinal and supracardinal channels that form a collar of veins encircling the aorta. The ventral portion of the circumaortic collar persists as the normal left renal vein. If the dorsal portion of this collar persists, then the left renal vein is posterior to the aorta, forming a RLRV [8,9].
The diagnostic methods for detecting IVC anomalies in previous reports were autopsy study, renal venography, color Doppler ultrasonography, computed tomography, and magnetic resonance imaging. With recent advances in computed tomography technology, MDCT has replaced conventional angiography and venography in most clinical conditions [6]. MDCT is a reliable, easily applicable, and noninvasive tool for demonstration of abdominal organs and vascular structures [6].
Left renal vein anomalies are generally classified into four types [5,6]. In type I, the ventral preaortic limb of the left renal vein is obliterated, but the dorsal retroaortic limb persists and joins the IVC in the orthotopic position [1,2,6,7]. Type II results from the obliteration of the ventral preaortic limb of the left renal vein, and the remaining dorsal limb turns into the RLRV. The left renal vein lies at the level of L4 to L5 and joins the gonadal and ascending lumbar veins before joining the IVC [1,2,6,7]. Type III is the circumaortic left renal vein or venous collar. This type is due to the persistence of subsupracardial and intersupracardial anastomoses and the dorsal limb of the left renal vein. If all small retroaortic veins that empty into the IVC are considered, the incidence of a circumaortic left renal vein could be as high as 16% [1,2,6,7]. In type IV, the ventral preaortic limb of the left renal vein is obliterated, and the remaining dorsal limb becomes the RLRV. Then, the RLRV courses obliquely and caudally behind the aorta to join the left common iliac vein [1,2,6,7]. The incidences of left IVC, double IVC, and retrocaval ureter are 0.2-0.5%, 0.3-2.8%, and 0.1% [1,2,8]. The incidences of RLRV type I, II, III, and IV are 0.3-1.9% [10,11], 0.4-0.9% [12,13], 1.5-8.7% [10,14], and 0.16% [6], respectively. RLRV type I, II, III, and IV in our cases occurred in 6, 2, 1, and 0 patients, respectively.
During the renal surgery, this kind of anatomical variation influences the technical feasibility of the operation. Failure to recognize these anomalies may lead to severe hemorrhage and severe renal damage [4]. Therefore, special attention is needed. Microscopic hematuria can be caused by increased pressure of the renal vein. The posterior "nutcracker phenomenon" occurs when a decreased space between the aorta and the vertebra causes compression of the RLRV. It is postulated that compression of the left renal vein leads to hematuria because of elevated pressure in the left renal vein, resulting in congestion of the left kidney and the venous communications [15-18]. It is well known that the gonadal, ascending lumbar, adrenal, ureteral, and capsular veins are potential collateral venous pathways in cases of renal vein compression or obstruction. These anomalous communication channels are responsible for hematuria. In addition, vascular dilatation of the afferent venous system can result from increased drainage pressure, which men can suffer from left-sided varicocele and women from pelvic congestion syndrome [19-21]. In our series, 3 patients with microscopic hematuria had type I, 1 had type II, and 1 had type III. Symptoms continued in all patients with microscopic hematuria, maybe because of increased pressure in the renal vein, that is, the posterior nutcracker phenomenon. In two patients with varicocele, symptoms spontaneously improved in one patient (type I), and the other patient (type II) resolved his problem after nephrectomy. In the two cases of type II with a left-sided UPJO, it was postulated that dilatation of the renal pelvis led to the UPJO directly posterior contacting the dilated left gonadal vein (Fig. 2C). In our cases, one patient with partial obstruction underwent pyeloplasty because of severe left flank pain, and the other patient underwent nephrectomy for a nonfunctioning kidney. In adult UPJO, the incidence of aberrant vessels is 35-39% [22-24]. Braun et al showed that 12 of 27 patients with UPJO had aberrant vessels. Three of 12 patients were related to dilated gonadal vessels and 1 patient had RLRV type III [25].
Cho et al reported the congenital absence of the IVC as a rare cause of pulmonary thromboembolism [26]. Gibo and Onitsuka reported a case of RLRV that showed left renal vein hypertension by pullback pressure measurement from the RLRV to the IVC [16]. Therefore, congenital anomalies of the venous system might easily induce the congestion of the renal bloodstream, and these anomalies might be the predisposing conditions of clinical symptoms such as hematuria, flank pain, and pelvic congestion.
Diagnosis of renal vein anomalies is important information in retroperitoneal surgery. Unawareness of this situation during retroperitoneal surgery can result in bleeding, nephrectomy, and even death [10]. Surgeons prefer the left renal vein in renal transplantation because of its longer length. Because of this, it is important to know the course of the left renal vein and whether it is pre-aortic or not. It is also important to be aware of anomalies of the renal vein for distinctive diagnosis of retroperitoneal tumors, retroperitoneal lymph node pathologies, and aortic dissection [10,16]. Before the renal surgery, careful reading of the preoperative RLRV imaging study helps to avoid fatal complications during the operation.
This study was limited by the fact that it was performed retrospectively, and the data were analyzed in selected patients who had urologic symptoms. Another limitation of our study is the relatively small sample size. As a consequence, we could not reveal the Korean incidence, follow-up results, concomitant diseases, or a casual relationship between RLRV and urologic symptoms. Therefore, additional confirmatory studies are required in the near future.
RLRV is usually asymptomatic. It may sometimes cause hematuria, flank pain, and vascular dilations (varicocele). The most common type of RLRV was type I. The microscopic hematuria due to RLRV is thought to be continued in the long-term follow-up. For patients with gross hematuria or flank and inguinal pain, individualized treatment such as conservative care, pyeloplasty, varicocelectomy, and nephrectomy should be selected. Widespread use of diagnostic MDCT in retroperitoneal diseases, particularly kidney tumors, can identify changes in the renal vascularization more easily, and thus allows urologists to plan a safe and less complicated surgery.
Figures and Tables
FIG. 1
Schematic illustration of the different types of retroaortic left renal vein anomalies. (A) type I, (B) type II, (C) type III, (D) type IV.
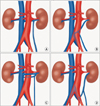
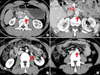
FIG. 2
Type I, II, and III of RLRV. (A) Type I of RLRV (arrow). (B) Type III of RLRV. The superior left renal vein (open arrow) and inferior left renal vein (arrow) crossed anterior and posterior to the aorta. (C, D) Ureteropelvic junction obstruction with type II of RLRV. Anteromedial crossing vessel (arrow in C) and joining IVC (arrow in D) at level L4. RLRV: retroaortic left renal vein, IVC: inferior vena cava.
References
1. Mayo J, Gray R, St Louis E, Grosman H, McLoughlin M, Wise D. Anomalies of the inferior vena cava. AJR Am J Roentgenol. 1983. 140:339–345.
2. Royal SA, Callen PW. CT evaluation of anomalies of the inferior vena cava and left renal vein. AJR Am J Roentgenol. 1979. 132:759–763.
3. Hayashi M, Kume T, Nihira H. Abnormalities of renal venous system and unexplained renal hematuria. J Urol. 1980. 124:12–16.
4. Thomas TV. Surgical implications of retroaortic left renal vein. Arch Surg. 1970. 100:738–740.
5. Cuéllar i Calàbria H, Quiroga Gómez S, Sebastia Cerquedà C, Boyé de la Presa R, Miranda A, Alvarez-Castells A. Nutcracker or left renal vein compression phenomenon: multidetector computed tomography findings and clinical significance. Eur Radiol. 2005. 15:1745–1751.
6. Karaman B, Koplay M, Ozturk E, Basekim CC, Ogul H, Mutlu H, et al. Retroaortic left renal vein: multidetector computed tomography angiography findings and its clinical importance. Acta Radiol. 2007. 48:355–360.
7. Shindo S, Kubota K, Kojima A, Iyori K, Ishimoto T, Kobayashi M, et al. Anomalies of inferior vena cava and left renal vein: risks in aortic surgery. Ann Vasc Surg. 2000. 14:393–396.
8. Bass JE, Redwine MD, Kramer LA, Huynh PT, Harris JH Jr. Spectrum of congenital anomalies of the inferior vena cava: cross-sectional imaging findings. Radiographics. 2000. 20:639–652.
9. Mathews R, Smith PA, Fishman EK, Marshall FF. Anomalies of the inferior vena cava and renal veins: embryologic and surgical considerations. Urology. 1999. 53:873–880.
10. Karkos CD, Bruce IA, Thomson GJ, Lambert ME. Retroaortic left renal vein and its implications in abdominal aortic surgery. Ann Vasc Surg. 2001. 15:703–708.
11. Minniti S, Visentini S, Procacci C. Congenital anomalies of the venae cavae: embryological origin, imaging features and report of three new variants. Eur Radiol. 2002. 12:2040–2055.
12. Hoeltl W, Hruby W, Aharinejad S. Renal vein anatomy and its implications for retroperitoneal surgery. J Urol. 1990. 143:1108–1114.
13. Kraus GJ, Goerzer HG. MR-angiographic diagnosis of an aberrant retroaortic left renal vein and review of the literature. Clin Imaging. 2003. 27:132–134.
14. Trigaux JP, Vandroogenbroek S, De Wispelaere JF, Lacrosse M, Jamart J. Congenital anomalies of the inferior vena cava and left renal vein: evaluation with spiral CT. J Vasc Interv Radiol. 1998. 9:339–345.
15. Lee SE, Park DS, Chung SY, Lee YT. Retroaortic renal vein. Korean J Urol. 2002. 43:84–86.
16. Gibo M, Onitsuka H. Retroaortic left renal vein with renal vein hypertension causing hematuria. Clin Imaging. 1998. 22:422–424.
17. Hohenfellner M, Steinbach F, Schultz-Lampel D, Schantzen W, Walter K, Cramer BM, et al. The nutcracker syndrome: new aspects of pathophysiology, diagnosis and treatment. J Urol. 1991. 146:685–688.
18. Rudloff U, Holmes RJ, Prem JT, Faust GR, Moldwin R, Siegel D. Mesoaortic compression of the left renal vein (nutcracker syndrome): case reports and review of the literature. Ann Vasc Surg. 2006. 20:120–129.
19. Graif M, Hauser R, Hirshebein A, Botchan A, Kessler A, Yabetz H. Varicocele and the testicular-renal venous route: hemodynamic Doppler sonographic investigation. J Ultrasound Med. 2000. 19:627–631.
20. Takahashi Y, Ohta S, Sano A, Kuroda Y, Kaji Y, Matsuki M, et al. Does severe nutcracker phenomenon cause pediatric chronic fatigue? Clin Nephrol. 2000. 53:174–181.
21. Scultetus AH, Villavicencio JL, Gillespie DL. The nutcracker syndrome: its role in the pelvic venous disorders. J Vasc Surg. 2001. 34:812–819.
22. Van Cangh PJ, Wilmart JF, Opsomer RJ, Abi-Aad A, Wese FX, Lorge F. Long-term results and late recurrence after endoureteropyelotomy: a critical analysis of prognostic factors. J Urol. 1994. 151:934–937.
23. Nakada SY, Wolf JS Jr, Brink JA, Quillen SP, Nadler RB, Gaines MV, et al. Retrospective analysis of the effect of crossing vessels on successful retrograde endopyelotomy outcomes using spiral computerized tomography angiography. J Urol. 1998. 159:62–65.
24. Keeley FX Jr, Moussa SA, Miller J, Tolley DA. A prospective study of endoluminal ultrasound versus computerized tomography angiography for detecting crossing vessels at the ureteropelvic junction. J Urol. 1999. 162:1938–1941.
25. Braun P, Guilabert JP, Kazmi F. Multidetector computed tomography arteriography in the preoperative assessment of patients with ureteropelvic junction obstruction. Eur J Radiol. 2007. 61:170–175.
26. Cho BC, Choi HJ, Kang SM, Chang J, Lee SM, Yang DG, et al. Congenital absence of inferior vena cava as a rare cause of pulmonary thromboembolism. Yonsei Med J. 2004. 45:947–951.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download