Abstract
Purpose
We evaluated the impact of renal tumor size on the oncologic and surgical efficacy of laparoscopic renal cryosurgery (LRC) according to our intermediate-term experience in Korea.
Materials and Methods
From June 2005 to October 2008, we enrolled 37 patients who underwent LRC for 40 renal tumors. Patients were stratified into four groups according to renal tumor size. Patients who presented with a maximum tumor diameter (MTD) of at least 1 cm but less than 2 cm were assigned to Group 1, those with an MTD equal to or greater than 2 but less than 3 cm were assigned to Group 2, those with an MTD equal to or greater than 3 but less than 4 cm were assigned to Group 3, and those with an MTD equal to or greater than 4 cm were assigned to Group 4. Oncologic and clinical outcomes in each group were compared.
Results
The four groups showed no statistically significant differences in preoperative variables, including age, sex, body mass index, American Society of Anesthesiologists scores, baseline renal function and hemoglobin, and length of hospital stay. Regarding surgical aspects, however, operation time, estimated blood loss, and postoperative complications were significantly increased in patients with larger tumors. Three patients in Group 3 required postoperative transfusions, and 1 patient in Group 4 required conversion to open renal cryosurgery. During the mean follow-up period of 31.6 months, radiologic evidence of tumor recurrence was found in only 2 patients in Group 4.
Conclusions
In this series, LRC for renal tumors smaller than 3 cm was conducted safely without radiologic evidence of tumor recurrence during intermediate-term follow-up. For tumors larger than 3 cm, however, the transfusion rate increased, and for renal tumors larger than 4 cm, the tumor recurrence rate increased significantly.
The increased use of cross-sectional images has led to renal tumor "stage migration," with the diagnosis of most masses occurring at an early stage [1]. Consequently, the size of newly diagnosed renal tumors has decreased, and the incidence of small renal tumors (less than 4 cm) has increased [2]. Surgical resection, predominantly by nephron-sparing surgery, is considered the standard of care for clinically localized renal cell carcinoma (RCC) because of the favorable prognosis associated with surgery and the relative ineffectiveness of systemic therapy. The advantages of this approach include the preservation of renal function and oncologic efficacy in terms of tumor recurrence and the long-term survival rate comparable to that of radical nephrectomy, which is the traditional treatment modality for renal tumors [3-5]. Along with advancements in minimally invasive approaches, laparoscopic partial nephrectomy (LPN) has been reported to have oncological efficacy comparable to that of open partial nephrectomy. LPN requires high laparoscopic dexterity, however, and, even for experienced hands, still requires a longer warm ischemic time and is associated with more complications than is open partial nephrectomy [6,7].
With the increasing application of minimally invasive surgery, several energy-based tissue-ablation technologies, including cryoablation and radiofrequency ablation, are being investigated. With these techniques, tissue is destroyed by in situ ablation instead of extirpation; thus, complications induced by renal ischemia and surgical excision can be avoided. The data suggest that of the alternative ablation modalities, cryoablation may result in significantly lower rates of local tumor progression compared with radiofrequency ablation techniques [8]. In particular, compared with a percutaneous approach, laparoscopic renal cryoablation (LRC) allows the surgeon to position the cryoprobe with greater precision by using intraoperative ultrasonography and to move adjacent organs away from the ablation site; also, bleeding can be monitored and controlled during the procedure [9].
However, for large renal tumors, an increased tumor recurrence rate and complications after LRC have recently been reported [10]. To validate the impact of renal tumor size on oncologic and surgical efficacy, we retrospectively evaluated the oncologic and surgical effects of LRC according to renal tumor size from our intermediate-term experience in Korea.
From June 2005 to October 2008, 37 patients in our institution underwent LRC for 40 renal tumors; the tumors were solid and were observed in radiologic findings, including computed tomography (CT) and magnetic resonance imaging (MRI). Indications for LRC included a high risk for partial nephrectomy or patient age greater than 70 years. High operative risk in our institution was defined as an American Society of Anesthesiologists (ASA) score of 3 or more. Among these inclusion criteria for LRC, absolute indications included bilateral tumors and patients with a solitary kidney or renal insufficiency. A patient with normal contralateral renal function but poor operability was defined as having an elective indication. Detailed information on all treatment methods, including radical nephrectomy, partial nephrectomy, and laparoscopic renal cryosurgery was given to all patients before surgery.
Patients were stratified into four groups according to renal tumor size as follows: Group 1 included patients presenting with tumors with a maximum tumor diameter (MTD) of at least 1 but less than 2 cm, Group 2 included those with tumors of at least 2 but less than 3 cm, Group 3 included tumors that were at least 3 but less than 4 cm, and Group 4 included tumors that were 4 cm or larger.
For those groups, perioperative variables, including ASA score, comorbidity, body mass index (BMI), baseline renal function, hemoglobin, tumor location and size, operative time, estimated blood loss, transfusion rate, duration of hospitalization, postoperative complications, postoperative renal function, and hemoglobin levels were evaluated retrospectively. Also, the results of the pathologic examination, which was conducted at the time of cryoablation and radiologic follow-up for recurrence and metastasis, were evaluated. Patients were initially evaluated at 1 and 3 months and then every 3 months during the first year. They were evaluated every 6 months during the second year, and then annually. Follow-up evaluations involved a medical history update, physical examination, blood pressure check, contrast-enhanced CT or MRI, chest radiography, measurement of serum electrolytes, and renal function tests. Lack of enhancement on CT or MRI along with stable or decreased tumor size was considered a sign of successful treatment. Recurrence was defined as increasing tumor size or lack of tumor shrinkage, as shown with image enhancement.
Postoperative biopsies were not performed routinely in this series and were performed only in cases of suspicious tumor recurrence as determined by radiological evaluation. The TNM staging system, revised by the International Union against Cancer (UICC, 2002), was used to define cancer stages.
A single surgeon performed all LRC procedures. Standardized instruments with three ports were used for the laparoscopic procedure. Tumors anterior to a horizontal line within the coronal plane through the renal hilum were generally approached transperitoneally. Tumors posterior to this line were approached retroperitoneally. Realtime intraoperative ultrasonography (Aloka Dynaview II, Americanlab, Miami, USA) was used in all cases to identify the lesion and to determine the position of the cryoprobe within the lesion. Intraoperative ultrasonography was also used to identify the extension of the ice-ball during the freezing cycle. The kidney was mobilized and the Gerota's fascia was opened to facilitate identification of the tumor. Fat overlying the tumor was retrieved for pathological examination. Depending on the tumor size before insertion of the cryoprobe, one or two needle biopsies were taken from the tumor. We used two types of cryoprobes, both with a diameter of 1.47 mm. A cryoprobe (Oncura, Plymouth Meeting, USA) that induced a -40℃ isothermal lesion measuring 8 mm in diameter was used from June 2005 to December 2006, and the IceRod™ (Oncura), which induced a -40℃ isothermal lesion measuring 14.5 mm in diameter and radiating from the ablation probe was used from January 2007 on. Before insertion into the kidney, the proper number and locations of the cryoprobes were carefully calculated on the basis of the preoperative imaging study and intraoperative ultrasonography. Two temperature probes were then inserted into the middle and peripheral margins of the tumor to ensure that the temperature within the tumor had dropped below -40℃, which is normally required for effective destruction of malignant renal tissue. A double-freeze cycle was applied in all cases, with an intervening thawing process. Hemostasis was achieved by filling the probe tract with fibrin glue (Baxter, Deerfield, USA) and Surgicel (Johnson & Johnson, Irvine, USA) after the second thaw to allow for safe removal of the cryoprobe.
SPSS 12.0 for Windows (SPSS Inc., Chicago, USA) was used for statistical analysis, and the Kruskall-Wallis and Pearson's chi-square test were used for analysis of the characteristics (operative time, oncological outcomes, perioperative complications, etc.) of each group.
The preoperative characteristics of each patient group are summarized in Table 1. The mean follow-up period was 31.6 (range, 18-49) months. There were 13 patients (15 tumors) in Group 1, 10 patients (10 tumors) in Group 2, 10 patients (10 tumors) in Group 3, and 4 patients (4 tumors) in Group 4. Mean tumor size was 1.5 cm (range, 1.0-1.7 cm) in Group 1, 2.14 cm (range, 2.0-2.4 cm) in Group 2, 3.28 cm (range, 3.0-3.7 cm) in Group 3, and 5.07 cm (range, 4-7.5 cm) in Group 4, and the difference was significant (p=0.001). However, mean age, sex, BMI, ASA score, baseline hemoglobin, and serum creatinine showed no statistically significant differences (Table 1).
Perioperative outcomes are summarized in Table 2. Operative time and estimated blood loss showed a significantly different increase in each group (p=0.045, 0.018), with an increasing trend in patients with larger tumor size. Mean operative time and estimated blood loss were 128 minutes and 78 ml in Group 1, 121 minutes and 90 ml in Group 2, 162 minutes and 116 ml in Group 3, and 205 minutes and 123 ml in Group 4. The results for mean hospital stay were similar (Table 2).
There were no postoperative complications, including postoperative transfusion, in Groups 1 and 2; however, three patients in group 3 required postoperative transfusion, and one patient in group 4 required open conversion for control of bleeding from the cryoprobe insertion site. Due to her religious creed, which prohibits blood transfusion, open cryoablation with meticulous bleeding control, instead of partial nephrectomy, was conducted for this patient. With the exception of these cases, none of the other patients experienced complications.
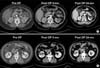
During the mean follow-up period of 31.6 months, radiologic evidence of tumor recurrence was found in 2 cases (5%), both in Group 4 (Fig. 1). One patient was a 65-year-old female with a history of cerebral infarction. She had a left solitary kidney and a tumor that measured 7.5 cm. One month after the initial LRC, contrast enhancement was observed by MRI at the surgical site (Fig. 1A). For this patient, open partial nephrectomy was conducted, with no findings of recurrence on radiological follow-ups after 9 months. Another patient was a 34-year-old female with a 4.0 cm endophytic enhancing mass in her left kidney. Due to her religion, which prohibits blood transfusion, she chose LRC instead of conventional operation. Contrast enhancement was still observed by CT performed 3 months after the initial LRC (Fig. 1B). We recommended another session of LRC, but after that the patient was lost to follow-up. All other patients, with the exception of these two, have remained free of recurrence or metastasis (Fig. 2).
Treatment options for small renal masses have expanded over the past decade. The increased use of cross-sectional imaging has led to "stage migration," with the diagnosis of most masses occurring at an early stage. For these small renal masses, open partial nephrectomy has replaced radical nephrectomy as the treatment of choice. As the result of its minimal invasiveness and promising oncologic outcome, LPN has gained interest; however, it is also associated with a higher rate of complications and a longer learning curve than that for open partial nephrectomy [11]. Ablation using a cryoprobe or radiofrequency probes offers the advantages of being a minimally invasive surgery with a significantly lower rate of complications when compared with LPN [12]. Furthermore, many of these masses are being diagnosed in elderly patients with co-morbidities, who are not good candidates for partial nephrectomy or a major surgical procedure.
In situ thermal destruction of renal masses through the creation of lethally cold temperatures offers a safer alternative. In addition, many early and intermediate-term reports have shown promising results for LRC. The goal of a cryoablative procedure is to inflict lethal freezing injury on the tumor volume while sparing normal healthy tissues. Although animal studies on renal cryosurgery have been reported since 1974 [8], reports on clinical application for human renal tumors are relatively recent. After the first human study on percutaneous cryosurgery was reported by Uchida et al in 1995 [9], open and laparoscopic surgeries were reported by Delworth et al in 1996 and Gill et al in 1998, respectively [11,12]. Since these promising initial reports, an increasing body of literature on this new modality has been released. In terms of safety, low incidence rates of renal failure, transfusion, and urinary leakage have been reported from clinical and animal studies so far [13-15]. Renal cryosurgery has been performed via open, laparoscopic, and percutaneous approaches by using cryoprobes with diameters ranging from 1.4 mm to 5.0 mm. In particular, laparoscopic renal cryosurgery, which has been more popular than the percutaneous approach, has the advantage of being able to identify the exact location of the cryoprobes and the lesion by use of intraoperative ultrasonography [16]. In addition, the laparoscopic approach allows for displacement of adjacent organs from the cyroablated site, thus decreasing the possibility of iatrogenic injuries.
Although no long-term follow-up data are currently available, most studies on this issue have reported promising oncologic control in early to intermediate-term follow-ups. Two published LRC series with a minimum follow-up period of 3 years demonstrated radiographically documented success rates of 97% and 96%, respectively [14]. Moinzadeh et al reported tumor recurrences in 3 of 56 tumors (5.4%) after LRC with a mean follow-up period of 36 months [17]. Schwartz et al reported recurrences in 2 patients among 50 tumors (4%) after LRC with a mean follow-up period of 10 months [18]. In this series with a mean follow-up period of 31.6 months, oncologic efficacy (radiologic recurrence of 5%) was comparable with that of previous studies. The rates of recurrence after LRC were also similar to those after LPN. Desai et al compared the outcome of 89 LRC procedures with those in 153 patients who underwent LPN [19]. The recurrence rate after LPN was 0.6% for an average follow-up period of 5.8 months and that after LRC was 3.0% for an average follow-up period of 24.6 months. Interestingly, estimated blood loss and the rate of postoperative complications were higher for LPN than for LRC (211 ml vs. 110 ml and 11.1% vs. 3.3%, respectively). Weight et al evaluated the efficacy of LRC in 109 tumors compared with 192 renal tumors treated by percutaneous radiofrequency ablation (RFA) [20]. The rate of radiological success, which was defined as no contrast enhancement on follow-up images for 6 months, was 85% after RFA and 90% after LRC. Pathological success rates, which were assessed 6 months after the initial ablation, were 64.8% for RFA and 93.8% for LRC.
Radiographic follow-up after cryoablation is the primary means of assessing the effect of treatment [21], and enhancement on post-contrast imaging is considered evidence of incomplete treatment of disease. Groups at some centers have performed biopsy after ablation for assessment of disease viability, whereas others have relied only on radiographic evaluation. Wright et al demonstrated excellent correlation between post-cryoradiographic findings and subsequent percutaneous biopsy of treated lesions, reporting that no lesion that failed to show enhancement on post-treatment imaging revealed evidence of a viable tumor [22]. In addition, biopsy can lead to false-negative results. On the basis of these findings, we did not use the staged postoperative histology for radiologically responding cases initially described by Desai and colleagues [19].
Although minor complications including transfusion occurred, major complications including urinary leakage, which is the most serious complication reported after renal cryosurgery, did not occur during our series. The low incidence of complications when performing LRC might be associated with the small diameter of the cryoprobe. Compared with larger-sized cryoprobes, the ultrathin 1.47 mm cryoprobes that were used in our series are associated with a relatively low risk of complications from bleeding or disastrous renal fracture [23]. Surgery-related problems, such as urine leakage, nephrectomy for bleeding, and adjacent organ damage have occurred in studies that used cryoprobes with a relatively large diameter. In contrast, no major complications were reported in three previous studies, including our own, that used ultrathin cryoprobes of 1.47 mm.
Another advantage of using ultrathin 1.47 mm cryoprobes is that multiple cryoprobes can be used for small lesions [24]. For reliable tumor destruction and for obtaining an adequate margin for the ice-ball, exact positioning of the cryoprobe in the center and peripheral margin of the tumor is of utmost importance. Use of multiple cryoprobes might hypothetically increase the efficiency of freezing by extending the coldest isothermal line, compared with the use of a single probe, and the distribution of the probes across the tumor might also compensate for an asymmetric tumor shape. However, in the case of a large tumor over 4 cm, despite an increase in the number of cryoprobes used, adjacent deep renal structures may cause interference in real-time identification and monitoring of the ice-ball. Thus, as shown in our results, proper consideration of the tumor size is the most important factor in achieving reliable oncologic control. In addition, the physician should keep in mind that increased tumor size also correlates with an increase in the rate of postoperative complications. This trend was comparable with results from Lehman et al, who reported a complication rate of 62% with LRC for larger renal tumors (over 3 cm), compared with no complications for their smaller counterparts [10]. Similar to our results, in their series, blood transfusions were the most common complication. In conclusion, our data illustrated the intermediate-term efficacy of LRC for treatment of small renal masses and a significantly increased complication rate and tumor recurrence rate in larger (over 4 cm) tumors.
Our experience with intermediate-term follow-up suggests that LRC was effective for renal tumors smaller than 3 cm without oncologic recurrence or complications. However, LRC for renal tumors larger than 3 cm was associated with increased transfusion rates. In addition, LRC for renal tumors larger than 4 cm was associated with a significant increase in tumor recurrence rates. In summary, although our data illustrate the intermediate-term efficacy of LRC for treatment of small renal masses, the complication rate and tumor recurrence rate were significantly increased in larger (over 4 cm) tumors. To ensure desirable oncologic efficacy, prudent patient selection for LRC will be imperative.
Figures and Tables
FIG. 1
(A) A 65-year-old female patient with a renal mass in the left solitary kidney that measured 7.5 cm. The arrow shows an ill-defined rim enhancement near the mass lesion at 1 month after left laparoscopic renal cryoablation by abdominal MRI scan (T1 images). (B) A 34-year-old female patient with a completely endophytic renal mass in the left kidney that measured 4.0 cm. The arrow shows a local recurrence near the mass lesion at 3 months after left laparoscopic renal cryoablation by abdominal pelvic CT scan.

FIG. 2
(A) A 63-year-old female patient with a 3.9 cm left renal cell carcinoma (RCC). The figure shows the decreased size of the treated RCC in the left kidney without a definite viable portion at 3 months and 24 months after left laparoscopic renal cryoablation by abdominal CT scan. (B) A 55-year-old male patient with a right RCC that measured 3.1 cm. The figure shows the decreased size of the treated RCC in the right kidney, without a definite viable portion at 3 months and 24 months after right laparoscopic renal cryoablation by abdominal CT scan.
References
1. Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of renal cell carcinoma. J Urol. 2001. 166:1611–1623.
2. Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology. 1998. 51:203–205.
3. Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cellcarcinoma: 10-year followup. J Urol. 2000. 163:442–445.
4. Patard JJ, Tazi H, Bensalah K, Rodriguez A, Vincendeau S, Rioux-Leclercq N, et al. The changing evolution of renal tumours: a single center experience over a two-decade period. Eur Urol. 2004. 45:490–493.
5. Wille AH, Tüllmann M, Roigas J, Loening SA, Deger S. Laparoscopic partial nephrectomy in renal cell cancer--results and reproducibility by different surgeons in a high volume laparoscopic center. Eur Urol. 2006. 49:337–342.
6. Allaf ME, Bhayani SB, Rogers C, Varkarakis I, Link RE, Inagaki T, et al. Laparoscopic partial nephrectomy: evaluation of long-term oncological outcome. J Urol. 2004. 172:871–873.
7. Gill IS, Matin SF, Desai MM, Kaouk JH, Steinberg A, Mascha E, et al. Comparative analysis of laparoscopic versus open partial nephrectomy for renal tumors in 200 patients. J Urol. 2003. 170:64–68.
8. Breining H, Helpap B, Minderjahn A, Lymberopoulos S. The parenchymal reaction of the kidney after local freezing. Urol Res. 1974. 2:29–31.
9. Uchida M, Imaide Y, Sugimoto K, Uehara H, Watanabe H. Percutaneous cryosurgery for renal tumours. Br J Urol. 1995. 75:132–136.
10. Lehman DS, Hruby GW, Phillips CK, McKiernan JM, Benson MC, Landman J. First prize (tie): laparoscopic renal cryoablation: efficacy and complications for larger renal masses. J Endourol. 2008. 22:1123–1127.
11. Delworth MG, Pisters LL, Fornage BD, von Eschenbacj AC. Cryotherapy for renal cell carcinoma and angiomyolipoma. J Urol. 1996. 155:252–254.
12. Gill IS, Novick AC, Soble JJ, Sung GT, Remer EM, Hale J, et al. Laparoscopic renal cryoablation: initial clinical series. Urology. 1998. 52:543–551.
13. Rukstalis DB, Khorsandi M, Garcia FU, Hoenig DM, Cohen JK. Clinical experience with open renal cryoablation. Urology. 2001. 57:34–39.
14. Gill IS, Novick AC, Meraney AM, Chen RN, Hobart MG, Sung GT, et al. Laparoscopic renal cryoablation in 32 patients. Urology. 2000. 56:748–753.
15. Janzen NK, Perry KT, Han KR, Kristo B, Raman S, Said JW, et al. The effects of intentional cryoablation and radio frequency ablation of renal tissue involving the collecting system in a porcine model. J Urol. 2005. 173:1368–1374.
16. Aron M, Gill IS. Renal tumor ablation. Curr Opin Urol. 2005. 15:298–305.
17. Moinzadeh A, Spaliviero M, Gill IS. Cryotherapy of renal masses: intermediate-term follow-up. J Endourol. 2005. 19:654–657.
18. Schwartz BF, Rewcastle JC, Powell T, Whelan C, Manny T Jr, Vestal JC. Cryoablation of small peripheral renal masses: a retrospective analysis. Urology. 2006. 68:1 Suppl. 14–18.
19. Desai MM, Aron M, Gill IS. Laparoscopic partial nephrectomy versus laparoscopic cryoablation for the small renal tumor. Urology. 2005. 66:5 Suppl. 23–28.
20. Weight CJ, Kaouk JH, Hegaty NJ, Remer EM, O'Malley CM, Lane BR, et al. Correlation of radiographic imaging and histopathology following cryoablation and radio frequency ablation for renal tumors. J Urol. 2008. 179:1277–1281.
21. Wyler SF, Sulser T, Ruszat R, Weltzien B, Forster TH, Provenzano M, et al. Intermediate-term results of retroperitoneoscopy-assisted cryotherapy for small renal tumours using multiple ultrathin cryoprobes. Eur Urol. 2007. 51:971–979.
22. Wright AD, Turk TM, Nagar MS, Phelan MW, Perry KT. Endophytic lesions: a predictor of failure in laparoscopic renal cryoablation. J Endourol. 2007. 21:1493–1496.
23. Cestari A, Guazzoni G, dell'Acqua V, Nava L, Cardone G, Balconi G, et al. Laparoscopic cryoablation of solid renal masses: intermediate term follwup. J Urol. 2004. 172:1267–1270.
24. Park MG, Kang SH, Cheon J. The initial experience with 3rd generation nephron-sparing cryoablation for renal tumor. Korean J Urol. 2007. 48:363–370.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download