Abstract
Purpose
Dendritic cell (DC)-based tumor vaccine is an attractive modality for the treatment of hormone-refractory prostate cancer (HRPC) because it has some efficacy and few side effects in patients with poor general conditions. The aim of this study was to establish which is the most effective DC vaccine for the treatment of HRPC. We compared DC vaccine sensitized with tumor lysate and a fusion vaccine of DCs and tumor cells.
Materials and Methods
The DU145 cancer cell line was purchased from the American Type Culture Collection. DCs were cultured from peripheral blood monocytes. Peripheral blood monocytes were cultured in RPMI 1640 medium supplemented with interleukin-4 (IL-4), granulocyte-macrophage colony-stimulating factor, and 10% fetal calf serum. Tumor necrosis factor-alpha was added on day 7 to support maturation. Functional activity was measured in three groups: the DC single-culture group, the DC culture group with DC vaccine sensitized with tumor lysates, and the DC culture group prepared with tumor fusion vaccine made from irradiated tumor cells and monocyte-derived DCs by the polyethylene glycol method.
Results
By FACS analysis, the rate of DC-tumor fusion vaccine was 20.3±3%. The IL-12 level produced by the DC-tumor fusion vaccine was significantly higher than that of DCs pulsed with tumor lysate (p<0.05). Also, the generation of interferon-γ by tumor-specific T cells in the DC-tumor fusion vaccine group was superior to that of DCs pulsed with tumor lysate (p<0.05). In addition, the T cells of the tumor lysate-pulsed DCs and tumor fusion vaccine had 1.6 and 2.5 times the functional activity, respectively, of the DC single-culture group in killing tumor cells in the cytotoxicity assay.
According to recent survival data for Korea, cancer-specific survival rates are 57.8%, 16.8%, and 10.1% in the 1st, 3rd, and 5th year, respectively, in patients with hormone refractory prostate cancer (HRPC) [1]. Dendritic cells are the strongest antigen-presenting cells. With the latest advances in immunology, new treatment methods using dendritic cells (DCs) have been proposed [2]. DCs stimulate not only memory T cell but also naive T cells and thereby induce appropriate immune responses [3]. For immunotherapy with DCs, the DCs should be sensitized before infusion to amplify their anti-cancer effects, which are specific to the corresponding tumor. Tumor antigens that are used for DC sensitization include peptides, proteins, DNA and RNA coding these antigens, and tumor lysates, which amplify the anti-cancer effects specific to the corresponding tumor before infusion. T cell reactions to sensitized DCs have been reported by many authors using various types of tumor cells [2,4-11]. Galea-Lauri et al reported that the immune responses occurring due to sensitization with a single antigen had a lower or a restricted degree of efficacy compared with tumor fusion vaccines [12].
DCs can be produced by culturing and proliferating monocytes that are extracted from the peripheral blood of normal healthy people. In the current study, we compared the feasibility and efficacy of DC fusion vaccines with those of DC only or DC sensitized with tumor lysate.
The hormone-refractory cell line DU145 was purchased from the American Type Culture Collection (Rockville, USA). RPMI 1640 (Gibco, Grand Island, USA), 10% fetal calf serum (FCS; Invitrogen, Carlsbad, USA), 100 units/ml penicillin, and 100 ug/ml penicillin-streptomycin solution (Gibco) were added as the basal medium for the culture of the DU145 cell line. The cells were cultured in an incubator at 37℃ and 5% CO2 by use of conventional cell culture methods.
Monocytes were isolated from peripheral blood mononuclear cells by density-gradient centrifugation with Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). The monocytes were cultured in RPMI 1640 medium to which 10% FCS was added at 37℃ for 2 to 3 hours. Then, the supernatant cells (T cells) were placed in a medium containing 90% heat-inactivated FCS to which 10% dimethyl sulfoxide (Sigma-Aldrich Corp., St. Louis, USA) was added.
The monocytes remaining on the floor of the flask were cultured in RPMI 1640 medium to which 40 ng/ml interleukin-4 (IL-4), 50 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF), and 10% FCS were added. A fresh medium to which cytokines were added was additionally provided at a 2-day interval. Tumor necrosis factor-alpha; 50 ng/ml; Centus Corporation, Emmeryville, USA) was added on day 7 after culture.
After culture, DCs were collected and then photographed by light microscopy. To examine the immune phenotypes, the cell surface was coated with antibodies such as CD3, CD83, and CD86, which were labeled with fluorescein isothiocyanate (FITC) and phycoerythrin (PE). This was followed by flow cytometry (Epics WL, Beckman Coulter, Brea, CA, USA).
To assist in the cell fusion, the cell line was irradiated with 150 Gy to induce apoptosis. After a 24-hour culture, the sample was used for the fusion.
Apoptosis of the HRPC cell line was assessed by the Annexin V binding assay kit. Cells were stained with FITC-Annexin V (BD-PharMingen, San Diego, USA) and propidium iodide (PI, 10 g/ml, Sigma). Cells undergoing early apoptosis were determined as the percentage of Annexin V+/PI- cells by FACScan with the Cell Quest 1.0 software package (BD, San Diego, USA).
Methods for fusing the nondependent prostate cancer cell line with DCs were based on the polyethylene glycol (PEG)-fusion protocol. DCs and the prostate cancer cell line were stained with cell marker materials, CD86-FITC and PKH-26-PE, respectively. Then, the cells were mixed at a ratio of 1:2. According to the PEG-fusion protocol, 1 ml of PEG 1500 (Applied Science, Penzberg, Germany) that was previously heated was added at 37℃ for 3 minutes, and 14 ml of PEG 1500 was additionally placed in a fusion medium, which was cultured at 37℃. The fusion rate of the fusion vaccine (CD86-FITC/PKH-26-PE) was measured by FACS analysis.
Briefly, DU-145 cells (2×106 cells/ml) were frozen at -80℃ for 20 min and were then thawed at 37℃ for 10 min. After three freeze-thaw cycles, the lysate was divided into aliquots and incubated with 1×106 DCs (lysate:DC ratio=3 tumor cells:DCs) overnight at 37℃ in complete media.
To acquire tumor-specific T-lymphocytes, the reaction was performed for T-lymphocytes corresponding to 10 to 50 times the number of DCs. Then, the sample was cultured in an X-vivo 15 medium (Bio Whittaker, Walkersville, USA) to which 20 ng/ml IL-7 and 100 pg/ml IL-12 (R&D Systems Inc., Minneapolis, USA) were added. On day 8, the reaction was performed again in an X-vivo 15 medium. Thereafter, on day 10, 20 IU/ml IL-2 was added. On day 10 after culture, T-lymphocytes were isolated and used for further laboratory procedures.
To analyze the functions of T-lymphocytes, T cells were placed in a 96-well plate at a fixed concentration of 1×105/well. Then, DCs were divided into the following groups:
Group 1, in which only DCs were added with T-lymphocytes in identical numbers; Group 2, in which DCs sensitized with tumor lysates were added with T-lymphocytes in identical numbers; and Group 3, in which DCs prepared with tumor fusion vaccines were added with T-lymphocytes in identical numbers. The cells were cultured in medium containing 10% FCS/RPMI, 400 ng/ml GM-CSF, and 16 ng/ml IL-4 for five days. After the addition of 5-bromo-2-deoxyuridine (BrdU), each well was isolated. The fused BrdU was measured by colorimetry.
IL-12 and interferon-gamma (IFN-γ) were measured by ELISA (Mabtech AB, Stockholm, Sweden). ELISA was performed in accordance with the manufacturer's instructions. Quantification was performed by using a VERSAmax microplate reader (Molecular Devices Corp., Sunnyvale, USA).
Cytotoxicity was measured in the three previously mentioned groups. In each group, the cells were placed in a 96-well plate, and the DU145 cell line was placed and cultured at 37℃ for 24 hours. Then, lactate dehydrogenase (LDH) that was leaked from the cell membrane of ruptured tumor cells was measured by use of the CytoTox-ONE assay (Promega Corp., Madison, USA).
Apoptosis was induced at a ratio of 10±2%. By use of flow cytometry, tumor-fused DCs were obtained at a ratio of 20.3±3% (Fig. 3).
The amount of BrdU that was used to measure the proliferation of T-lymphocytes showed an absorption rate of 0.32±0.02 (143%) in the group of DCs sensitized by using tumor lysates compared with the T cell single group as the control. In the group of tumor-fused DCs, the absorption rate was 0.67±0.07 (297%; p<0.05) (Fig. 4).
The concentration of IL-12 was 6.3±0.16 pg/ml (range, 6.1-6.5 pg/ml), 25.3±0.25 pg/ml (range, 25.1-25.6 pg/ml), and 55.6±0.19 pg/ml (range, 55.3-55.8 pg/ml) in the DC single group, tumor lysate sensitized group, and DC-tumor fusion group, respectively (Fig. 5).
The concentration of IFN-γ was 15±1.22 pg/ml (range, 13.5-16.5 pg/ml), 40±1.70 pg/ml (range, 38-42 pg/ml), and 92.5±0.79 pg/ml (range, 91.5-93.5 pg/ml) in the DC single group, tumor lysate sensitized group, and DC-tumor fusion group, respectively (Fig. 6).
The concentration of LDH was 1,532 relative fluorescence units (RFU) in the group in which DCs were solely cultured, 2,483.2 RFU in the group in which DCs were sensitized with tumor lysates, and 3,923.8 RFU in the group in which DCs were fused with tumor cells (Fig. 7).
DCs are potent antigen-presenting cells able to induce primary immune responses. DCs capture and process antigens into peptides. DCs then present the antigens to T cells and B cells through MHC class I and II molecules [3]. Peptides, proteins, DNA and RNA coding these antigens, and tumor lysates have been used to sensitize DCs [4]. An alternative approach to increasing antitumor immunity is the use of fusions of DCs and tumor cells [12]. In this approach, a broad spectrum of tumor-associated antigens, including those known and unidentified, are processed endogenously and presented by MHC class I and II pathways in the context of co-stimulatory signals.
A major issue for the application of DC-tumor cell fusion vaccines is the development of compatible and reproducible procedures that can be readily translated to the clinical setting. Of equal concern is the availability of sufficient tumor material for the preparation of tumor cells. In this study, we integrated several features important for the clinical development of DC-tumor cell fusion vaccines: (1) a simple process for the generation of DCs, (2) PEG protocols that permit fusion of sufficient numbers of DCs and tumor cells, and (3) the application of tumor cells derived from allogenic cell lines.
In the past, it was difficult to purely isolate DCs and to culture them in a massive amount. It has been reported, however, that DCs can effectively be cultured in massive amounts from human bone marrow, fetal cord blood CD34+ hematopoietic stem cells, and CD14+ monocytes present in the peripheral blood [13-16]. Methods for producing tumor fusion vaccines include PEG and electrical stimulation [17]. Compared with electrical stimulation, for which specific types of equipment are required, the PEG methods are relatively simpler. In comparison with the reported fusion rate, they are also cost-effective.
In our study, the rate of observed allogenic DC-tumor cell fusion vaccines generated by PEG protocols was 20.3±3%. This rate was relatively lower than other previous reports using PEG. In general, the fusion rate has been reported to be 12% to 60% [17,18]. According to Lundqvist et al, following an experiment using three different types of HRPC cell lines, there was a consistently higher degree of treatment response, compared with non-hormone-dependent cells, in tumor fusion vaccines that were prepared by using a single type of cell line [18]. These findings may be evidence that the same immune responses might also occur in patients with HRPC who are assumed to have an equivalent degree of antigenicity in the clinical application of allogenic tumor-fused cell vaccines.
Our study applied pre-irradiated tumor cells to induce apoptosis in fusion vaccine preparations; radiation doses varied between 30 Gy and 200 Gy. Increasing doses of irradiation would induce higher levels of apoptosis, and high-level cell death has been observed in populations of DU 145 cells over time after exposure to 200 Gy. Determination of an appropriate radiation dose is necessary, especially because tumor cells are well known to display variability in their radiosensitivity. In this study, the 150 Gy dose was the most effective for the DC-DU 145 cell fusion vaccine.
Concentrations of IL-12 and IFN-γ were measured to be more than two times higher to a statistically significant extent in group 3 than in group 2. These results well illustrated that DCs and tumor-specific T cells stimulated with DC-tumor fusion had a higher degree of functional activity. We used the LDH assay in the cytotoxic test, which confirmed a substantial extent of tumor cell destruction. Compared with the control group, the cytotoxic results in group 2 and group 3 were statistically significant. In particular, cytotoxicity was significantly higher in group 3 than in group 2. In group 1, nonsensitized DCs might hinder rather than help the proliferation of T cells. The absorption rate of BrdU was 0.19±0.02 (84.1%) lower than in the control group.
The DC-tumor fusion rate in our study was 20.3%, which is lower than that of other reports. The DC-tumor fusion vaccine was functionally active and more effective in stimulating DCs and T cells in HRPC cells than were DCs sensitized with tumor lysate. The DC-tumor fusion vaccine was also superior to tumor lysate-sensitized DCs for the cytotoxicity of HRPC cells. We suggest the possibility of treatment with the allogenic tumor fusion vaccine in prostate cancer in which there is a lower degree of variability in tumor antigens. Further experimental studies are warranted to obtain higher rates of fusion for clinical application. In addition, other attempts such as the manipulation of cofactors should be made to further enhance the functional activity of DCs.
Figures and Tables
 | FIG. 1Photograph of dendritic cells on day 7. Aggregates of dendritic cells developed from adherent peripheral blood monocytes under the influence of GM-CSF and IL-4 (×1,200, PENTAX A10, Pentax Corporation, Japan, OLYMPUS CK2, Olympus Optical Co., Ltd. Japan). GM-CSF: granulocyte-macrophage colony-stimulating factor, IL-4: interleukin-4. |
 | FIG. 2Phenotypic characterization of immature and mature DCs by flow cytometry. (A) Phenotypes of immature DCs. (B) Phenotypes of mature DCs. Immature dendritic cells were generated from peripheral blood mononuclear cells (PBMCs) of healthy donors by culturing with IL-4 and GM-CSF. Differentiation into mature DCs was achieved by the addition of inflammatory cytokine cocktail for 24 hours. Mature DCs have a higher CD83 and CD86 count than do immature DCs. DCs: dendritic cells, IL-4: interleukin-4, GM-CSF: granulocyte-macrophage colony-stimulating factor. |
 | FIG. 3Efficacy of PEG hybridization of dendritic cells and the DU145 tumor cell line. Before hybridization, tumor cells were stained with PKH26PE and DCs were stained with monoclonal antibody against CD86FITC. FACS analysis showed that 20.3% of DC-tumor hybrids were obtained (circled area). Tumor cells were irradiated at 150 Gy and pooled with mature DCs at a 1:2 ratio. DC: dendritic cell. |
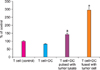 | FIG. 4Mixed lymphocyte reaction of tumor lysate-pulsed DCs and DC-tumor hybrid. Cells were co-cultured with 105 allogenic T cells/well in 96-well plates for 5 days. For 15-20 hours before harvesting, 1 uCi of H3-thymidine was added to each well. Cells were harvested and incorporated BrdU was measured colorimetrically (a: p<0.05). DC: dendritic cell. |
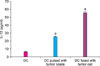 | FIG. 5Dendritic cell function estimated by IL-12 assay. Supernatants from tumor lysate-pulsed DCs and DC-tumor hybrid were analyzed for production of IL-12 by ELISA (a: p<0.05). DC: dendritic cell, IL-12: interleukin-12. |
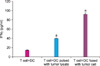 | FIG. 6Generation of IFN-γ-producing tumor-specific T cells. T cells re-stimulated by co-culture with tumor lysate-pulsed DCs and DC-tumor hybrid were analyzed for production of IFN-γ by ELISA (a: p<0.05). IFN-γ: interferon-gamma, DC: dendritic cell. |
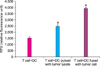 | FIG. 7Generation of cytotoxicity of tumor lysate-pulsed DCs and DC-tumor hybrid. T cells stimulated by DC cells pulsed with DU145 tumor lysate and DC/DU145 hybrid cells were cocultured with DU145 cells in a 96-well plate for 24 hours at 37℃. The CytoTox-ONE assay measured the release of lactate dehydrogenase (LDH) from cells with a damaged membrane (a: p<0.05). DC: dendritic cell. |
References
1. Ha HK, Yun CJ, Lee SS, Shin DG, Lee W, Lee ZZ, et al. Survival rates and related factors in men with hormone-refractory prostate cancer. Korean J Urol. 2009. 50:649–655.
2. Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med. 1996. 2:52–58.
3. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998. 392:245–252.
4. Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998. 4:328–332.
5. Kundu SK, Engleman E, Benike C, Shapero MH, Dupuis M, van Schooten WC, et al. A pilot clinical trial of HIV antigen-pulsed allogeneic and autologous dendritic cell therapy in HIV-infected patients. AIDS Res Hum Retroviruses. 1998. 14:551–560.
6. Gong J, Chen D, Kashiwaba M, Kufe D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med. 1997. 3:558–561.
7. Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998. 4:594–600.
8. Murphy G, Tjoa B, Ragde H, Kenny G, Boynton A. Phase I clinical trial: T-cell therapy for prostate cancer using autologous dendritic cells pulsed with HLA-A0201-specific peptides from prostate-specific membrane antigen. Prostate. 1996. 29:371–380.
9. Segel LA, Jäger E, Elias D, Cohen IR. A quantitative model of autoimmune disease and T-cell vaccination: does more mean less? Immunol Today. 1995. 16:80–84.
10. Lotze MT, Shurin M, Davis I, Amoscato A, Storkus WJ. Dendritic cell based therapy of cancer. Adv Exp Med Biol. 1997. 417:551–569.
11. Bendandi M, Gocke CD, Kobrin CB, Benko FA, Sternas LA, Pennington R, et al. Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor against lymphoma. Nat Med. 1999. 5:1171–1177.
12. Galea-Lauri J, Darling D, Mufti G, Harrison P, Farzaneh F. Eliciting cytotoxic T lymphocytes against acute myeloid leukemia-derived antigens: evaluation of dendritic cell-leukemia cell hybrids and other antigen-loading strategies for dendritic cell-based vaccination. Cancer Immunol Immunother. 2002. 51:299–310.
13. Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007. 117:1195–1203.
14. Banchereau J, Steinman RM. Dendritic cells and the control immunity. Nature. 1998. 392:245–252.
15. Reid CD, Stackpoole A, Meager A, Tikerpae J. Interactions of tumor necrosis factor with granulocyte-macrophage colony-stimulating factor and other cytokines in the regulation of dendritic cell growth in vitro from early bipotent CD34+ progenitors in human bone marrow. J Immunol. 1992. 149:2681–2688.
16. Caux C, Vanbervliet B, Massacrier C, Dezutter-Dambuyant C, de Saint-Vis B, Jacquet C, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J Exp Med. 1996. 184:695–706.
17. Scott-Taylor TH, Pettengell R, Clarke I, Stuhler G, La Barthe MC, Walden P, et al. Human tumour and dendritic cell hybrids generated by electrofusion: potential for cancer vaccines. Biochim Biophys Acta. 2000. 1500:265–279.
18. Lundqvist A, Palmborg A, Bidla G, Whelan M, Pandha H, Pisa P. Allogeneic tumor-dendritic cell fusion vaccines for generation of broad prostate cancer T-cell responses. Med Oncol. 2004. 21:155–165.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download