INTRODUCTION
Anatomical prostatectomy was first reported by Walsh in 1983.1 After the advent of urological laparoscopic surgery, Schussler and Kavoussi reported on the initial experience of laparoscopic removal of the prostate and concluded that it was too difficult and offered no advantage over open surgery.2 Guilonneau and Vallencien from France rejuvenated laparoscopic surgery by reporting their success in laparoscopic prostatectomy.3 Soon, European surgeons adopted the laparoscopic technology, but in the United States and other countries, the learning curve to laparoscopic surgery was a major obstacle to overcome. In 2000 the United States Food and Drug Administration (FDA) approved the human use of the da Vinci™ surgical robot system (Intuitive Surgical, Sunnyvale, USA). Afterward, as the result of pioneering by Menon and associates at Henry Ford Hospital in Detroit, robotic-assisted laparoscopic prostatectomy (RALP) was introduced and offered to the urological community.4 Since then, the use of the surgical robot in the treatment of localized prostate cancer has been a dramatic change in various parts of the world, including the United States and the Republic of Korea. Robotic technology offers better defined surgical anatomy and improved surgical maneuverability, resulting in improved surgical outcome and surgeon comfort in the laparoscopic prostatectomy.
The da Vinci™ robot system has advantages such as 7-degrees of freedom including the operator's grip, a 3-dimensional vision, intuitive motion, and the filtration of unwanted physiologic tremors; it allows ease of intracorporeal dissection and suturing secondary to the wristed and articulating instrumentation. The da Vinci™ robot system, however, has some disadvantages; it is still expensive, it requires training, and it is devoid of tactile feedback. After the introduction of the surgical robot in July 2005, various urological procedures, including radical prostatectomy, partial nephrectomy, nephrectomy, cystectomy, and nephroureterectomy, have been performed in Korea.5-8 This review presents the evolution of the surgical technique and the current status of RALP.
EVOLUTION OF THE SURGICAL STEPS
After the initial use of RALP in Korea at Yonsei University on July 15, 2005, a total of 18 da Vinci™ robot systems had been installed in the Republic of Korea as of January 2009. Surgeons have experimented with various techniques for robotic prostatectomy. As a result of the better visualization and resulting improved understanding of the surgical anatomy, the fascial coverings around the prostate have been better appreciated, which has resulted in different surgical techniques and continued refinements to the procedure.
1. Conventional nerve sparing technique
Menon et al suggested the Vattikuti Institute Prostatectomy (VIP) technique, which is based on the nerve-sparing prostatectomy technique established by Walsh.1,4 The incision is made in the anterior prostatic fascia parallel to the running direction of the neurovascular bundle and is extended to the lateral side of the prostate with a dissection of the prostatic fascia. Thus, the posterolateral neurovascular bundles are sharply dissected.
2. Endopelvic fascia saving (veil of aphrodite) technique
Also revolutionized by Menon and associates,4 in this technique, while preserving the lateral prostatic fascia, the dissection is performed along the posterolateral aspect of the prostate with verification of the layer and is advanced up to the apex, and the neurovascular bundles are exposed and separated from the prostate. This can be accomplished right after breaking through the posterolateral aspect of the prostate about 1.5-2.5 cm in length, where the branches of the nerve and vessels are passing by. The avascular layer is exposed up to the apex, and the dissection is advanced to the posterior area of the dorsal vein complex. This technique has been applied after the procedural learning curve was overcome in selected cases with localized prostate cancer in the preoperative MRI, CT, and WBBS and a low Gleason's score.
3. Ultradissection technique (lateral dissection of the prostate and bladder neck)
Lateral dissection of the prostate and bladder neck was first introduced by Gaston from France in 2006.9 Conventional dissection of the bladder neck starts from the anterior aspect of the bladder neck and resects the posterior aspect of the bladder neck at the midline, and then approaches the Denonvilliers fascia, seminal vesicles, and vas deferens. The ultradissection technique is a modification of the lateral dissection technique, with dissection of the avascular layer among the bladder, prostate, and periprostatic tissue, reaching the seminal vesicles and vas deferens first, dissecting the bladder neck from the prostate except the urethra, and finally cutting the urethra.9
4. Extraperitoneal approach
Similar to the extraperitoneal approach of laparoscopic prostatectomy, RALP can be performed without violating the peritoneal cavity. The layer between the rectus abdominis and posterior fascia is dissected with the fingers, a balloon dilator is put into the space, and then the space of Retzius can be obtained to perform the robotic surgery.10
STEP-BY-STEP PROCEDURE
1. Patient position and port placement
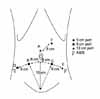
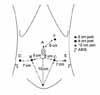
After the induction of anesthesia, the patient is placed in a modified lithotomy position, with all pressure points padded. The arms are tucked at the patient's side. The chest is secured with the placement of a horizontal three-inch tape, as well as Velcro straps. At this point, the stability of the patient in steep Trendelenburg should be tested. The patient is prepped and draped. A 20 Fr. Foley catheter is inserted on the field. Port configuration is shown in Fig. 1 (six ports). A Veress needle is utilized through a 12 mm supraumbilical incision for the entry of the 1st port (A: camera port) for the transperitoneal approach. Following a drop test, pneumoperitoneum is obtained at 20 mm Hg. Two 8 mm ports (B [patient's right side], C [patient's left side]) for the robot instruments are placed at 8 cm laterocaudal to the camera port and 15 cm cranial to the pubis symphysis. An 8 mm port for the 4th arm is placed at 8 cm laterocaudal to the B port in a direction toward the anterior superior iliac spine (ASIS). A 12 mm port (E) is placed for an assistant instrument at 8 cm laterocaudal to the C port in a direction toward the ASIS. Last, a 5 mm port (F) for assistant's suction is placed at approximately 8 cm cranial to the midline of the A and C ports. For a small pelvis, this port configuration is adjusted (Fig. 2). The most lateral ports (D and E ports) are placed horizontally 7 cm apart from the B and C ports, respectively. This port adjustment prevents the D and E ports from being interrupted by the ASIS. Following the placement of the ports, pneumoperitoneum is decreased to 15 mm Hg and maintained throughout the procedure. The patient is tilted in a 30° Trendelenburg position and the robot is docked in place.
2. Surgical technique of RALP
1) Exposure of extraperitoneal space and lymph node dissection: Dissection is started with the peritoneum medial to the vas deference with 0° lens and monopolar scissors (surgeon's right hand). The surgeon's left hand holds the bipolar forceps. The median umbilical ligament is transected as cranial as possible to avoid the peritoneal flap from interrupting the surgeon's view. The extraperitoneal space is exposed, followed by release of colonic attachment, allowing further mobilization of the bladder. Lymph node dissection is performed bilaterally in the external iliac, obturator, and infraobturator area. Preprostatic fat is also removed until the endopelvic fascia is identified. However, unlike in the conventional method, the endopelvic fascia is not excised.
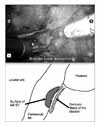
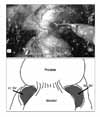
2) Bladder neck and seminal vesicle dissection (Modified ultradissection): A lens is switched to 30° for a bladder neck dissection. A Foley catheter is mobilized in and out to help to identify the prostatovesical junction. The bladder is retracted cranially with the 4th arm. Ultradissection of the bladder neck as described by Gaston's group is performed in a modified manner.9 Detrusor muscle fibers are identified, and the lateral border of the bladder neck is separated until it reaches the surface of the seminal vesicle (Fig. 3). Unlike in the original method by Gaston, the seminal vesicle is not dissected further and nerve sparing is not performed at this point. Following bilateral dissection of the bladder neck, the detrusor muscle is well appreciated (Fig. 4). Then, the bladder neck is transected. This technique allows preservation of the bladder neck even with prostates with a large median lobe and in previous transurethral resected cases. The vasa deferentia are transected and the seminal vesicles are mobilized. Retraction of the seminal vesicles with the 4th arm 45° superomedially facilitates this dissection.
3) Nerve sparing (lateral endopelvic fascia sparing technique): Neurovascular bundles are preserved in selected patients with low-risk prostate cancer. The vasa deferentia and seminal vesicles are retracted upward by the 4th arm. A lens is switched back to 0° for posterior dissection of the prostate. Denonvilliers fascia is sharply excised transversally and perirectal fat is visualized. Further blunt dissection of the space between Denonvilliers fascia and the rectum is carried out to the apex of the prostate. Then, the lateral pedicles are controlled by titanium clips. In a localized low-grade, low-volume prostate cancer patient, the neurovascular bundles are preserved maximally in intrafascial fashion, which is also called the "Veil of Aphrodite technique" described by Menon's group.4 Articulated robotic scissors are used to incise the prostatic fascia anterior and parallel to the neurovascular bundles. After the correct plane is entered, most dissection is performed in a relatively avascular plane. In selected patients in intermediate to high-risk groups, interfascial or extrafascial nerve sparing is performed accordingly.
4) Transection of DVC and urethra, urethrovesical anastomosis, and puboperineoplasty: The DVC is fulgurated with bipolar forceps and sharply transected or ligated with 2-0 absorbable sutures. The puboprostatic ligament is spared. A plane between the urethra and DVC is gently developed to expose the anterior urethral wall. The urethra is completely mobilized at this point because the posterior apex was dissected earlier. The urethra is sharply transected and the prostate is removed and placed in a medium-sized plastic entrapment bag. Posterior fixation stitching is performed with 3-0 polyglactin 910 (Vicryl, Ethicon, Somerville, NJ) for posterior wall reconstruction. Vesicourethral suturing follows. A suture is prepared by tying two (17 cm+17 cm) 3-0 poliglecaprone 25 (Monocryl, Ethicon, Somerville, NJ) on a UR6 needle back to back to create a double-armed suture with a pledget of knots. The suture is started at the posterior bladder wall at the 4 o'clock position outside-in with the aid of a 16 Fr. silastic Foley catheter. Running suture is finished at the 11 o'clock position. Another suture completes the contralateral side of the vesicourethral anastomosis, starting with outside-in on the bladder neck. The running suture continues to incorporate the DVC, and the two sutures are tied together. Surgicels (Ethicon, Somerville, NJ) are placed in the bilateral border of the bladder neck for hemostasis. The puboprostatic collar and bladder are incorporated by 3-0 Monocryl running sutures (puboperineoplasty). Surgicels and Fibrin sealant (Baxter, Deerfield, IL) are applied around the vesicourethral junction. The urethrovesical anastomosis is tested for any leaks with 100 ml of saline. The Foley catheter is exchanged for a 16 Fr silastic Foley catheter, and the balloon is inflated to 10 cc.
5) Retrieval of specimen and completion of surgery: A 5 mm suction drain is placed through the left 5 mm port. The prostate is removed via a supraumbilical incision.
6) Postoperative care: The drain is removed when the volume is <200 ml/day. Patients are usually discharged on postoperative day three.
RESULTS
During the last few years, RALP has become a viable option in urological practice. Although open radical prostatectomy is the gold standard for the treatment of localized prostate cancer, RALP has been reported to have similar outcomes in operative time, blood loss, continence, potency, and oncological results.
2. Estimated blood loss and transfusion rate
Retropubic radical prostatectomy has been associated with higher estimated blood loss and transfusion rates. Pneumoperitoneum during laparoscopic prostatectomy and RALP exerts a tamponade effect that results in decreased blood loss. Transfusion rates after RALP have been reported to be up to 0.5% and estimated blood loss to be from 75 to 664 ml.4,11 The mean estimated blood loss in our institution is 250 ml per case.
3. Continence
Reporting of continence outcome has not been standardized. Different measures (interviews, questionnaires) and various definitions of continence are used, and physician and patient perspectives of continence are greatly varied. Strict criteria of leak and pad-free status should be used in further studies to accurately compare results. Patel and coworkers reported continence rates of 47%, 28%, 89%, 92%, and 98% at 1, 3, 6, 9, and 12 months, respectively,12 and Menon et al. reported 95.2% at 12 months after lateral prostatic fascia-saving RALP in over 2,000 patients.13 Their definition was no pads or a single pad for security purposes. Our results reported in 2008 after 237 cases by use of questionnaire and focused interview were 42.6%, 61.6%, and 79.89% at 3, 6, and 12 months, respectively.14 Our criteria were validated by a voiding dysfunction specialist and perhaps closely resemble the actual patient experience. The continence reporting of RALP suggests a tendency for earlier recovery of continence, but no prospective, randomized studies assessing the impact of surgical technique are available to deduce a valid conclusion.
4. Potency
Erectile dysfunction is an inevitable consequence of surgical trauma induced by all forms of prostatectomy. Therefore, efforts have been made to minimize this trauma by reducing injury to neurovascular bundles, thermal or mechanical injury to the bundles, or sparing periprostatic structures. In one of the largest series reported by Menon and coworkers at Vattikuti Institute in Detroit, they used a self-administered Sexual Health Inventory for Men (SHIM) questionnaire preoperatively and at 12 months postoperatively. Recovery of a SHIM score greater than 21 was defined as recovery of normal erections. Using this criterion, 70% of men with a preoperative SHIM score greater than 21 reported normal erections at 12 months and 50% of them required erection-enhancing medications.15 Patel reported 87.7% of patients with normal erections after a minimum follow-up of 3 months.16
In our Korean experience, even though it is too early to analyze, preoperative SHIM scores are generally lower than US reports and subsequently the return of normal erection is expected to be lower than previously reported.
5. Oncological outcomes
The rates of positive surgical margins vary widely from 2% to 59%.17 Patel et al. reported the positive margin rates for T2, T3a, T3b, and T4 tumors to be 5.7%, 29%, 20%, and 33%, respectively.12 The distribution of positive surgical margins was apex, 23%; bladder neck, 14.5%; posterolateral, 36.7%; and multifocal, 26%. In our experience with ultradissection, the positive surgical margin on the base of the prostate was significantly reduced by modified ultradissection to 1.0% from 8.0% (p=0.02). For any techniques of RALP to be a truly gold standard practice, oncological results should be proven to not be compromised.
CLINICAL CONSIDERATIONS
RALP is a rapidly growing minimally invasive surgical approach. It is becoming a standard alternative to both open and laparoscopic surgical treatment for localized prostate cancer, especially in the United States. The advantages of RALP are the same as for other laparoscopic procedures, including less postoperative pain, a shorter convalescence, less bleeding, and better cosmesis. The robotic approach has added more advantages provided by the enhanced 3-dimensional view with maximal magnification of x12 and the EndoWrist technology, which allows 7 degrees of freedom compared with the 4 degrees of freedom of a non-robot-assisted laparoscopic approach (Fig. 5). However, the overall clinical outcome depends not only on precise maneuvers, but also on a better understanding of the anatomy.
There are disadvantages of RALP, such as a longer set up time due to positioning and docking of the robot and expensive initial and disposable costs. We used a structured approach to establishing a laparoscopic radical prostatectomy program, converting to robotic surgery in July 2005. Currently, it is our preferred method for treating localized prostate cancer. Our technique has been refined. Since December 2005, we have switched the nerve sparing technique in patients with low risk and low volume disease from the conventional interfascial technique described by Walsh1 to the intrafascial so-called "Veil technique."4 This is the high anterior release technique, aiming at separating the prostate capsule from the prostatic fascia from the posterolateral direction to preserve the nerves that run along the lateral side. Other technical modifications include the posterior fixation stitch, the so-called Rocco stitch,18 which has been used since September 2007. Their idea is based on the fact that the musculofascial plate, which comprises the striated sphincter, Denonvilliers fascia, and the dorsal aspect of the prostate, acts as a suspensory system for the prostatomembranous urethra. Therefore, its division during radical prostatectomy results in the loss of the posterior cranial insertion of the sphincter, the caudal displacement of the sphincteric complex, and a prolapse of the perineum. The Rocco stitch is the posterior reconstruction of the rhabdosphincter (RS), aiming at a rapid recovery of continence by joining the posterior median raphe with the connected dorsal wall of the RS to the residuum of the Denonvilliers fascia and to suspend it to the posterior wall of the bladder, 1-2 cm cranially, and dorsally to the bladder neck. Their recent report demonstrated a significant rapid recovery of continence, 74.2% versus 25% at catheter removal and 83.8% versus 32.3% at 30 days after surgery with or without this technique, respectively, in patients who underwent laparoscopic transperitoneal bladder neck-sparing radical prostatectomy. We favor this technique also for other aspects. The bladder neck comes closer to the urethra by this stitch and it enables a tension-free anastomosis. It also makes a vesicourethral anastomosis technically easier.
We approximate the puboprostatic collar and bladder at the end of the procedure (puboperineoplasty). It is a simple procedure and takes only 3-5 minutes. This is aimed at better hemostasis and better continence. The arcus tendineus plays an important role in continence in men and women. The preservation of the puboprostatic collar including the Arcus tendineus has been demonstrated to restore early continence in men undergoing robotic prostatectomy.19 In that study, they reconstructed the puboprostatic collar by approximating the remaining arcus tendineus and distal triangular plate to the bladder neck. In 50 patients, the continence rate was 29% in the first week, 62% at 6 weeks, 88% at 12 weeks, and 95% at 16 weeks after catheter removal.
Modified ultradissection has been used since November 2007. This is the technique first described by Curto and Gaston in 2006.9 Their technique differs from others in several steps: (1) not opening the reflection of the endopelvic fascia, (2) the puboprostatic ligaments are not divided, (3) the DVC is not ligated before the removal of the prostate, (4) dissection of bladder neck is initiated bilaterally and it circles around the urethra that is preserved, and (5) the lateral pelvic fascia is not incised anteromedially and parallel to the NVBs but it is reflected off the prostate up to the apex. There are several advantages of this technique. Preservation of the endopelvic fascia allows preservation of a small sphincteric accessory nerve branch situated between the lateral part of the prostate and the levator ani muscle. Puboprostatic ligament sparing has the potential for a rapid recovery of continence. Bilateral dissection of the bladder neck enables better bladder neck preservation. It is difficult to preserve a nice bladder neck, especially in patients with a large median lobe, by means of the conventional laparoscopic antegrade approach. This technique also allows precise dissection of the bladder neck. In the original technique by Gaston, left nerve sparing follows left-side bladder neck dissection, and then right-side bladder neck dissection and transection of the bladder neck is performed. We perform bladder neck transection prior to the nerve-sparing procedure. We feel that this modified technique is easier and faster. Safety is a major issue for any new technology, and our intraoperative complication rate during our robotic experience was 2.7%, which is comparable with the results of open or laparoscopic radical prostatectomy.20
The incidence of prostate cancer is lower in Asian countries than in other countries. However, it is increasing because of PSA screening and increased public awareness. Pelvic surgery including radical prostatectomy is difficult in a small pelvis because of the small working space. The body habitus of an especially small pelvis is common in the Asian population. In our experience, the most challenging step in the small pelvis is vesicourethral anastomosis. The EndoWrist technology with articulated instruments allows the surgeon successful anastomosis even in a small pelvis. Port configuration is also important (Fig. 1). We do not change the A, B, or C port configuration even in a small pelvis. An 8 cm distance between the camera port and the 2nd and 3rd arm is required to avoid interruption between the robotic arms. The D (8 mm port for 4th arm) and E (assistant's 12 mm port) ports can be adjusted instead. We place the D and E ports 1 cm medial to the conventional port configuration in a small pelvis. The interruption between the robotic arms and the ASIS is avoided in this manner. The extraperitoneal approach is also launched in our institution. In the approach, although the A port (camera port) is placed in the infraumbilical incision, the other port configuration is similar to the one in a transperitoneal approach. We believe this modification with the D and E ports enables RALP in a small pelvis without any surgical disadvantages.
CONCLUSIONS
RALP is a safe, effective, and reproducible technique for the surgical treatment of localized prostate cancer. In most patients, it can be performed in a reasonable operative time of approximately 3 hours, including bilateral lymphadenectomy, with acceptable perioperative, oncological, and functional outcomes.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download