Abstract
Purpose
We evaluated the feasibility of robot-assisted laparoscopic radical cystectomy (RARC) with pelvic lymph node dissection (PLND), especially extended PLND (ePLND), during our initial experience with this technique.
Materials and Methods
From August 2007 to March 2009, prospective data were obtained from the 21 consecutive patients who underwent RARC with PLND at Korea University Hospital. Data included baseline characteristics, perioperative variables, pathological outcomes, and complications. Evidence of the lymph node yield curve was examined by using linear regression to compare the number of lymph nodes obtained.
Results
Among 21 patients who underwent RARC, 13 had ileal conduit urinary diversion and 8 had orthotopic neobladder. Standard PLND (sPLND) was performed in the first 15 patients, and ePLND was performed in the more recent 6 patients. The mean total operative time was 515.5±145.1 minutes, and the mean estimated blood loss was 346.8±205.9 ml. The mean time for PLND was 106.7±25.2 minutes in patients with ePLND and 72.1±14.1 minutes in patients with sPLND (p=0.001). All patients had negative surgical margins. The mean number of retrieved nodes was 23.5±12.8 (range, 8-50) in all patients: 38.6±10.8 (range, 29-50) in ePLND and 15.7±12.2 (range, 8-21) in sPLND.
Radical cystectomy is the gold standard option for patients with muscle-invasive bladder cancer and for those with recurrent, high-grade non-muscle-invasive bladder cancer [1]. About 25% of patients have lymph node metastasis according to pathologic evidence at the time of radical cystectomy, although preoperative evaluation does not show metastatic lymphadenopathy [2]. In that aspect, it has been suggested that pelvic lymph node dissection (PLND) has an important role in disease staging and a therapeutic or even curative effect in selected patients. Herr described that survival was improved regardless of positivity of lymph node metastasis when a higher number of lymph nodes were removed [3]. Leissner et al reported that the disease-free interval was increased with more lymph nodes retrieved, regardless of the presence or absence of lymph node metastases [4]. In an attempt to maximize lymph node removal during radical cystectomy, the extent of pelvic lymphadenectomy has been questioned. Poulsen et al reported an improved 5-year recurrence-free survival with extended PLND (ePLND) beginning at the bifurcation of the aorta and including the common and external iliac vessels, presacral nodes, and obturator fossa [5].
The introduction of laparoscopy in urology has been expanding in the field of PLND. In 1991, Schuessler et al reported the first laparoscopic PLND for prostate cancer, which was safe and feasible [6]. Finelli et al reported that laparoscopic pelvic lymphadenectomy for bladder cancer was feasible and efficient [7], but laparoscopic procedures have prolonged operative times, significant technical difficulties, and a difficult learning curve [8].
Recently, a robot-assisted laparoscopic technique has emerged that allows surgeons to more readily overcome the difficult learning curve. The robot-assisted laparoscopic surgical system has a 3-dimensional imaging system and the patented Endowrist® technology to enable meticulous dissection and easy handling of laparoscopic instruments. Robot assistance reduces the difficulty in performing laparoscopic procedures, particularly in comparison with pure laparoscopic pelvic surgery. The robot-assisted laparoscopic technique has been applied to pelvic lymphadenectomy. Woods et al reported that robotic ePLND was safe and feasible in the management of bladder cancer [9]. Guru et al also reported that robot-assisted PLND was safe and lymph node yield was oncologically acceptable [10].
To our knowledge, however, there has been no report on the feasibility and efficiency of robot-assisted laparoscopic pelvic lymphadenectomy in Korea. Herein we prospectively evaluate whether robot-assisted laparoscopic pelvic lymphadenectomy can be performed without compromising morbidity and mortality during radical cystectomy, especially ePLND.
From August 2007 to March 2009, 21 patients who had muscle-invasive bladder cancer or high-grade, recurrent non-muscle-invasive bladder cancer underwent robot-assisted laparoscopic radical cystectomy (RARC) with PLND at Korea University Hospital.
All patients were identified by a pathologic diagnosis from a previous transurethral resection of a bladder tumor. Preoperative laboratory examinations and imaging studies for staging work-up, including abdominopelvic computed tomography and simple chest radiography, were performed for all patients. Perioperative data were also prospectively collected, including operative times, estimated blood loss, and presence of complications. In the pathology department, direct visualization and palpation by pathologists were used to identify the lymph nodes (LNs), with no use of fat clearing solutions. The analyzed parameters for operative time were total operative time and PLND time. Postoperative pathologic data were also obtained on the bladder and retrieved LNs. Descriptive statistics such as the mean in the case of numeric variables and the relative frequency in the case of categorical variables were computed by patient or by group. To further examine the curve associated with the lymph node yield (LNY) as the numbers of patients operated upon increased, linear regression and Spearman correlation analysis were used. All statistic data were analyzed by SPSS version 12.0 (SPSS Inc, Chicago, USA).
All of the operations were performed by a single surgeon who had performed 5 cases of open radical cystectomy during a 2-year urologic oncology fellowship when starting RARC. In terms of the laparoscopic approach, he had no experience with pure laparoscopic pelvic surgery including prostatectomy or cystectomy when starting RARC. The Da Vinci-S™ surgical system (Intuitive Surgical, Sunnyvale, USA) was used. All patients were prepared in the same way for open radical cystectomy, including bowel preparations and antibiotics preparations from 2 days before the operations. Leg compression stockings were worn at 06:00 of the operation day.
In the operating room, general anesthesia was achieved, and a nasogatric tube and a 18 Fr urethral catheter were inserted. The patient's lower extremities were laid in the extended lithotomy position, and the arms were adducted and padded sufficiently while the operation table was placed in the deep Trendelenburg position. Pneumoperitoneum was created by using a 10 mm port for the camera, installed by the Hasson technique at 4 cm above the umbilicus. Two 8 mm trocars for the robotic arms were inserted about 8 cm lateral to the camera port at the level of 2 cm above the umbilicus, and one more trocar for the third arm was inserted at 8 cm lateral to the second arm port. Two 11 mm ports for an assistant were placed at 3 cm superior to the right iliac crest and superiorly right to the camera port while the robot was docked between the patient's legs (Fig. 1).
A 0° lens was used for PLND. Lymphadenectomy was performed with the following anatomical boundaries: the hypogastric nerve posteriorly, the genitofemoral nerve laterally, the inferior mesenteric artery proximally, and the circumflex iliac vein and the node of Cloquet distally (Fig. 2). The descending and sigmoid colons were released from the side wall to allow access to the left iliac vessels. Careful dissection was needed using a robotic dissector with bipolar electrocautery in the left hand, and robotic shear with monopolar electrocautery in the right hand. The large cephalad lymphatics were ligated with Hem-o-lok® clips (Weck, Research Triangle Park, USA) to prevent lymphatic leak, and the small cephalad lymphatics were coagulated by bipolar electrocautery. The caudal side was ligated with Hem-o-lok® clips only when a blood vessel was encountered. The dissection was performed along the common iliac artery from the inferior mesenteric artery to the bifurcation of the common iliac artery. The genitofemoral nerve was identified overlying the psoas muscle, and the lymphatics were divided at that location, creating the lateral border. The dissection was carried distally as the hypogastric artery and vein were exposed, and the superior vesical artery was isolated, clipped, and divided in preparation for division of the vascular pedicles to the bladder. The lymphatics overlying the external iliac artery and presacral area were split. The node of Cloquet was isolated, and the lymphatics overlying the external iliac vein were reflected medially and cephalad, exposing the pelvic side wall and the obturator nerve. The same dissection was carried out on the right side, starting at the common iliac artery. All the retrieved lymph nodes were sent to the pathology department in 6 packets: common iliac, external iliac, presacral, obturator, genitofemoral, and paraaortic. The boundaries of standard PLND (sPLND) were the obturator nerve posteriorly, the genitofemoral nerve laterally, the common iliac vessels proximally, and the node of Cloquet distally. sPLND was performed technically in the same way as ePLND. ePLND was performed during the late phase of this study, whereas sPLND was during the initial phase.
RARC was performed after the PLND was completed. All urinary diversions were created extracorporeally, and the neovesicourethral anastomoses were robotically performed with redocking of the robotic instruments.
Of the 21 patients, 20 patients were male and 1 was female. The mean age of all patients was 61.5 years (range, 44-78) and mean body mass index (BMI) was 25.7 kg/m2 (range, 21.2-30.3). The mean American Society of Anesthesiologists (ASA) score of the patients was 2.29 (range, 1-3). No patients had preoperative lymph node metastasis on the abdominopelvic CT scan. Preoperatively, the clinical stages of the patients were 7 (33.3%) with superficial cancer (T1 and carcinoma in situ), 12 (57.1%) with muscle-invasive cancer (T2), and 2 (9.5%) with advanced stages over T2. Two patients who had advanced stages over T2 had undergone 3 cycles of platinum-based neoadjuvant chemotherapy. Nineteen patients had transitional cell carcinoma in the pathologic examination from transurethral resection of bladder tumor (TURBT). Of the 2 patients with non-urothelial-cell carcinoma from TURBT, one had primary adenocarcinoma of the bladder and another had urothelial carcinoma with small cell carcinoma. There were no significant differences in the baseline characteristics of patients between the sPLND and ePLND groups (Table 1).
Among the 21 patients, 13 had an ileal conduit and 8 had a Studer-type orthotopic neobladder. sPLND was performed in 15 patients, and ePLND in 6. The mean total operative time was 515.5 minutes (range, 310-720): 516.4 minutes (range, 310-700) in the sPLND group and 650.0 minutes (range, 430-720) in the ePLND group (p=0.107). The average PLND time was 74.7 minutes (range, 50-145); the mean times for the sPLND group and the ePLND group were 72.1 minutes (range, 50-100) and 106.7 minutes (range, 70-145), respectively (p=0.005). The mean estimated blood loss was 346.8 ml (range, 90-590) in all patients: 348.7 ml (range, 90-590) and 341.7 ml (range, 270-410) in the sPLND and ePLND groups, respectively (p=0.696) (Table 2). Postoperative complications occurred in 9 patients (42.9%). Major complications, belonging to Memorial Sloan-Kettering Cancer Center (MSKCC) grade 3 [11,12], occurred in 3 patients (14.3%). Minor complications, belonging to MSKCC grade 1 or 2, occurred in 6 patients (28.6%). In the sPLND group, 6 patients (40%) suffered from postoperative complications, 3 patients (20%) with major criteria and 3 patients (20%) with minor criteria. Three patients (50%) in the ePLND group suffered from postoperative complications, all of which were minor (Table 3). There was no intraoperative major vascular injury, vital organ injury, or open conversion. There was no patient with formation of lymphocele or lymphedema. The mean hospital stay was 17.3 days (range, 8-38) in all patients: 18.2 days (range, 8-38) in the sPLND group and 15.3 days (range, 11-19) in the ePLND group (p=0.785). The average time to flatus was 3.3 days (range, 2-33): 3.9 days (range, 2-33) and 2.1 days (range, 3-5) (p=0.967) in the sPLND and ePLND groups, respectively (Table 2).
Pathologic stages were T0, T1, and CIS in 7 patients (33.3%); T2 in 9 patients (42.8%); T3 in 3 patients (14.3%); and T4 in 2 patients (9.5%). All patients had negative surgical margins. The average numbers of retrieved LNs were 23.5 (range, 8-50) in all patients: 15.7 (8-21) in patients with sPLND and 38.6 (29-50) in patients with ePLND (p=0.001) (Table 4). As operative experience increased, the LNY for all patients improved with no significant increase in operative duration (p=0.008) (Fig. 3). The LNY also especially improved, as operative cases increased, for ePLND cases (p=0.005) (Fig. 3). The correlation between the number of cases and LNY was very strong (rho=0.943). Three patients had positive LNs retrieved by sPLND. The mean postoperative follow-up periods were 8.9 months (1-21), and one patient with LN metastasis died from lung metastasis of bladder cancer. The overall survival rate was 95.2% (20 of 21 patients), and the disease-specific survival rate was also 95.2%.
The initial reports on the role of PLND were from poor outcomes of patients with local pelvic recurrence after simple cystectomy [13]. Colston and Leadbetter reported a cadaveric study and found that 25% had metastatic disease localized to the lymphatic tissue of the pelvis and retroperitoneum [14]. Whitmore and Marshall reported that a survival benefit was possible when lymphadenectomy was combined with cystectomy [15]. Lymphadenectomy during radical cystectomy might serve both a diagnostic and a therapeutic purpose. Despite the development of imaging techniques, however, the significant lack of accuracy and the discrepancy between clinical and pathologic staging is evident [16,17]. To overcome these limitations, several new techniques, such as positron emission tomography (PET), single photon emission computed tomography (SPECT), etc, are currently being studied [18,19]. But, currently available imaging techniques are not yet accurate enough to evaluate LN involvement. Therefore, for more accurate pathologic information to improve patient survival, there has been increased interest in the role of lymphadenectomy.
Several studies indicate that the number of LNs retrieved is the prognostic factor in both LN-positive and LN-negative patients. Herr et al reported the results of 322 patients undergoing radical cystectomy and PLND: 258 (80%) node-negative patients and 64 (20%) node-positive patients [20]. Survival for both LN-negative and LN-positive patients was longer when a greater number of LNs were removed. Leissner et al reported the results of 447 patients undergoing racial cystectomy and PLND. In that study, 65% of the patients with 16 or more LNs had 5 or more years of disease-free survival, although only 51% of the patients with 15 or more LNs had 5 or more years of disease-free survival. There was a similar difference in tumor-specific survival [4].
The number of pathologically assessed LNs is influenced by several factors, including (1) the boundaries of LND, (2) the pathologists' diligence in searching for and preparing the LNs, and (3) the way the specimen is submitted for pathologic assessment. The boundaries of LND are considered to be one of the most important factors determining the number of LNs removed. The limits of the LND may have the greatest impact on the number of LNs removed during cystectomy. An extended template has been suggested to increase the number of LNs removed. ePLND is recently favored, which approaches the level of the aortic bifurcation or inferior mesenteric artery. In one multicenter study [21], the mean total number of LNs removed was 43.1 by ePLND. Poulsen et al reported that more LNs were removed in extended dissection (25 LNs versus 14 LNs in standard dissection) [5]. Bochner et al confirmed these findings, reporting a significantly greater number of nodes removed with an extended dissection when compared with a more standard dissection, that is, 36.5 versus 8.5, respectively [22]. The way a specimen is submitted for pathologic assessment also affects LN yield. Bochner et al reported that to facilitate nodal evaluation, separate nodal packets should be submitted intraoperatively by the urologists [22]. In our study, the extents of ePLND were the hypogastric nerve posteriorly, the genitofemoral nerve laterally, the inferior mesenteric artery and common iliac vessels proximally, and the circumflex vein and the node of Cloquet distally. This followed the trend in currently performed lymphadenectomy [10]. We also used separate LN packets, instead of the en bloc method of LN submission. The 3 packets in the standard procedure included (1) the genitofemoral nerve to external iliac artery package below the bifurcation of common iliac artery, (2) the node of Cloquet and external iliac vein package, and (3) the obturator package proximally to the hypogastric artery. The 5 packets in the extended procedure included (1) the 3 packets of the standard procedure, (2) the inferior mesenteric artery to common iliac package, and (3) the presacral package. The mean number of retrieved LNs was 38.6±10.8 (range, 29-50) in patients in the ePLND group and 15.7±12.2 (range, 8-21) in patients in the sPLND group (p=0.013). The number of retrieved LNs in this study is comparable to that in the previously mentioned studies.
Many studies suggest that ePLND is beneficial to survival in patients undergoing radical cystectomy, although controversy over its usefulness is still present. In 1982, Skinner reported the first experience with ePLND [23]. Approximately 30% of LN-positive patients undergoing ePLND had long-term survival. Poulsen et al reported a modest improvement in the 5-year recurrence-free survival rate for the ePLND group (62% versus 56% for the sPLND group) [5]. Dhar et al compared the data from patients treated by radical cystectomy and limited PLND or ePLND [24]. The 5-year recurrence-free survival rate of patients with LN-positive disease was 7% for limited dissection and 35% for ePLND. The 5-year recurrence-free survival rate for pT2N0 cases was 67% for limited PLND and 77% for ePLND, and the respective percentages for pT3N0 were 23% and 57%. In those studies, ePLND might have enabled more accurate staging and removal of preoperatively undetected LN metastases. This could improve the survival of patients with histopathologic LN-positive and LN-negative disease. We can't present the long-term disease-free survival results of the present study, but both the overall survival rate and the disease-free survival rate with short-term follow-up were 95.2% (20 of 21 patients) in this series of 21 patients. However, we expect promising results, considering the number of LNs retrieved compared with the other studies.
Although ePLND may have beneficial effects on tumor treatment, the risks for morbidity and mortality associated with ePLND must be carefully evaluated. The extended procedure has been shown to increase operative duration by 1 to 1.5 hours compared with the standard procedure [7,25]. Brossner et al analyzed whether the morbidity from radical cystectomy increased with ePLND [26]. The patients in the ePLND group had an operation time that was around 60 minutes longer than that for patients in the sPLND group. However, no significant differences were found in perioperative morbidity, early complications, or the need for blood transfusion. Poulsen et al reported a similar mortality and lymphocele formation between the ePLND group and the limited PLND group [5]. Leissner et al also reported no significant difference in the formation of lymphoceles or lymphedema [4]. We reported similar results for perioperative morbidity. Although ePLND increased the operation time by approximately 30 minutes, there were no early complications related to PLND and no lymphocele or lymphedema formation. Postoperative complications occurred in 9 patients (42.9%). Of 15 patients in the sPLND group, postoperative complications occurred in 6 patients (40%). Three patients (50%) in the ePLND group suffered from postoperative complications. There was no significant difference in the postoperative complication rate between the groups. Major complications, belonging to MSKCC grade 3, occurred in 3 (14.3%) of 21 patients, who were all in the sPLND group and not the ePLND group. One patient had undergone explorative laparotomy due to mechanical ileus, and another patient had suffered from leakage of an ureterointestinal anastomosis. The patients were managed by percutaneous nephrostomy catheterization and ureteral stenting. Arteriosclerosis obliterans occurred in the other patient, who had received medical treatment and recovered. Minor complications, belonging to MSKCC grade 1 or 2, occurred in 6 (28.6%) of 21 patients: 3 patients (20%) in the sPLND group and 3 patients (50%) in the ePLND group. The minor complications included cases of prolonged ileus, acute renal failure, postoperative delirium, and leakage of urethral anastomosis (Table 3).
Minimally invasive surgery is now beginning to gain acceptance as a viable alternative to selected open oncological procedures in urology [27]. There are several reports on the safety and efficacy of laparoscopic PLND and radical cystectomy. Haber and Gill reported the results of 37 patients who underwent laparoscopic PLND with radical cystectomy [28]. Limited PLND was used in the initial 11 patients, with a median yield of 6 nodes, whereas ePLND was used in the subsequent 26 patients, with an increased median yield of 21 nodes. Overall survival was better when ePLND was used (76% vs 50% in limited PLND). Finelli et al reported the feasibility and efficacy of laparoscopic ePLND [7]. The median number of nodes retrieved was 3 in the limited PLND group and 21 in the ePLND group. Indeed, laparoscopic cystectomy can be safely performed by a few highly skilled laparoscopic surgeons.
To overcome the technical difficulty of laparoscopic surgery, robot assistance was introduced as a way to shorten the learning curve. The use of robot assistance has been expanding in the field of radical cystectomy and PLND, especially ePLND. In a prospective study comparing robot-assisted radical cystectomy and laparoscopic radical cystectomy [29], the mean number of LNs removed among RARC patients who had undergone ePLND was 22.3, whereas the mean number was 16.5 for the laparoscopic radical cystectomy patients. Guru et al reported the feasibility of robot-assisted ePLND with RARC in 67 patients; the mean number of LNs retrieved in that study was 18, and the frequency of patients with a LNY of >13 also increased overall with experience [10]. Also, robot-assisted PLND required a mean of 46 minutes, and 1 postoperative vascular complication occurred. Woods et al reported similar results [9]. The mean total operative time of 27 patients undergoing RARC with ePLND was 400 minutes, and average blood loss was 277 ml. The mean number of LNs removed was 12.3. In our study, the difference between the operation time for ePLND and sPLND (106.7 vs 72.1 minutes) was significant (p=0.005). However, the differences in mean estimated blood loss, total operative time, hospital stay, and time to flatus were not statistically significant. The average numbers of retrieved LNs were 23.5±12.8 (range, 8-50) in all patients, 15.7±12.2 (range, 8-21) in patients undergoing sPLND, and 38.6±10.8 (range, 29-50) in patients undergoing ePLND. These results are comparable to previously mentioned studies of RARC and PLND. Moreover, the more cases we experienced, the more LNs were removed. This may be influenced by the improvement in surgical skill and the fact that most patients undergoing ePLND were operated on during the late phase of this series.
Our study has several limitations. First, most cases of extended dissection were performed during the late phase of this series. Because the authors did not experience pure laparoscopic pelvic surgery at the time of starting this series, sufficient experience to overcome the learning curve was required for the robot-assisted laparoscopic pelvic surgery. Second, we cannot assess the recurrence-free survival and disease-free survival because of the short follow-up interval. Similar outcomes for survival are expected, however, considering the previously mentioned literature. Because of these several limitations, more experiences are required to adequately evaluate and validate this procedure as an appropriate surgical and oncologic option for the management of bladder cancer.
Perioperative data and oncologic features suggest that RARC with PLND is feasible. From the viewpoint of PLND, especially ePLND, the robotic procedure is a safe and effective procedure with acceptable morbidity and good oncologic results, even when performed by a laparoscopy-naïve surgeon. Large randomized controlled studies with long-term follow-up are required to confirm the oncologic results and survival benefit of this procedure.
Figures and Tables
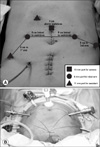 | Fig. 1The port placement for RARC with PLND. (A) Port sites and incisions for extracorporeal urinary diversions. All trocars, except for one assistant port, are placed on a concentric line from the umbilicus. (B) Port placement before docking of the robotic instrument. RARC: robot-assisted laparoscopic radical cystectomy, PLND: pelvic lymph node dissection. |
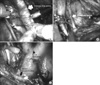 | Fig. 2Landmark of extended pelvic lymph node dissection. (A) Genitofemoral nerve to external iliac artery, (B) obturator nerve, (C) inferior mesenteric artery and aortic bifurcation. |
 | Fig. 3Lymph node yield (LNY) curve: The patients in the extended pelvic lymph node dissection (ePLND) group had a larger LNY than did the patients in the standard PLND group. |
References
1. Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001. 19:666–675.
2. Madersbacher S, Hochreiter W, Burkhard F, Thalmann GN, Danuser H, Markwalder R, et al. Radical cystectomy for bladder cancer today--a homogeneous series without neoadjuvant therapy. J Clin Oncol. 2003. 21:690–696.
3. Herr HW. Extent of surgery and pathology evaluation has an impact on bladder cancer outcomes after radical cystectomy. Urology. 2003. 61:105–108.
4. Leissner J, Hohenfellner R, Thüroff JW, Wolf HK. Lymph-adenectomy in patients with transitional cell carcinoma of the urinary bladder: significance for staging and prognosis. BJU Int. 2000. 85:817–823.
5. Poulsen AL, Horn T, Steven K. Radical cystectomy: extending the limits of pelvic lymph node dissection improves survival for patients with bladder cancer confined to the bladder wall. J Urol. 1998. 160:2015–2019.
6. Schuessler WW, Vancaillie TG, Reich H, Griffith DP. Transperitoneal endosurgical lymphadenectomy in patients with localized prostate cancer. J Urol. 1991. 145:988–991.
7. Finelli A, Gill IS, Desai MM, Moinzadeh A, Magi-Galluzzi C, Kaouk JH. Laparoscopic extended pelvic lymphadenectomy for bladder cancer: technique and initial outcomes. J Urol. 2004. 172:1809–1812.
8. Türk I, Deger S, Winkelmann B, Schönberger B, Loening SA. Laparoscopic radical cystectomy with continent urinary diversion (rectal sigmoid pouch) performed completely intracorporeally: the initial 5 cases. J Urol. 2001. 165:1863–1866.
9. Woods M, Thomas R, Davis R, Andrews PE, Ferrigni RG, Cheng J, et al. Robot-assisted extended pelvic lymphadenectomy. J Endourol. 2008. 22:1297–1302.
10. Guru KA, Sternberg K, Wilding GE, Tan W, Butt ZM, Mohler JL, et al. The lymph node yield during robot-assisted radical cystectomy. BJU Int. 2008. 102:231–234.
11. Martin RC 2nd, Brennan MF, Jaques DP. Quality of complication reporting in the surgical literature. Ann Surg. 2002. 235:803–813.
12. Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992. 111:518–526.
13. Leadbetter WF, Cooper JF. Regional gland dissection for carcinoma of the bladder; a technique for one-stage cystectomy, gland dissection, and bilateral uretero-enterostomy. J Urol. 1950. 63:242–260.
14. Colston JA, Leadbetter WF. Infiltrating carcinoma of the bladder. J Urol. 1936. 36:669.
15. Whitmore WF Jr, Marshall VF. Radical total cystectomy for cancer of the bladder: 230 consecutive cases five years later. Trans Am Assoc Genitourin Surg. 1962. 54:20–32.
16. Mehrsai A, Mansoori D, Taheri Mahmoudi M, Sina A, Seraji A, Pourmand G. A comparison between clinical and pathologic staging in patients with bladder cancer. Urol J. 2004. 1:85–89.
17. Shariat SF, Palapattu GS, Karakiewicz PI, Rogers CG, Vazina A, Bastian PJ, et al. Discrepancy between clinical and pathologic stage: impact on prognosis after radical cystectomy. Eur Urol. 2007. 51:137–149.
18. Anjos DA, Etchebehere EC, Ramos CD, Santos AO, Albertotti C, Camargo EE. 18F-FDG PET/CT delayed images after diuretic for restaging invasive bladder cancer. J Nucl Med. 2007. 48:764–770.
19. Sherif A, Garske U, de la Torre M, Thörn M. Hybrid SPECT-CT: an additional technique for sentinel node detection of patients with invasive bladder cancer. Eur Urol. 2006. 50:83–91.
20. Herr HW, Bochner BH, Dalbagni G, Donat SM, Reuter VE, Bajorin DF. Impact of the number of lymph nodes retrieved on outcome in patients with muscle invasive bladder cancer. J Urol. 2002. 167:1295–1298.
21. Leissner J, Ghoneim MA, Abol-Enein H, Thüroff JW, Franzaring L, Fisch M, et al. Extended radical lymph-adenectomy in patients with urothelial bladder cancer: results of a prospective multicenter study. J Urol. 2004. 171:139–144.
22. Bochner BH, Herr HW, Reuter VE. Impact of separate versus en bloc pelvic lymph node dissection on the number of lymph nodes retrieved in cystectomy specimens. J Urol. 2001. 166:2295–2296.
23. Skinner DG. Management of invasive bladder cancer: a meticulous pelvic node dissection can make a difference. J Urol. 1982. 128:34–36.
24. Dhar NB, Klein EA, Reuther AM, Thalmann GN, Madersbacher S, Studer UE. Outcome after radical cystectomy with limited or extended pelvic lymph node dissection. J Urol. 2008. 179:873–878.
25. Stein JP. Lymphadenectomy in bladder cancer: how high is "high enough"? Urol Oncol. 2006. 24:349–355.
26. Brossner C, Pycha A, Toth A, Mian C, Kuber W. Does extended lymphadenectomy increase the morbidity of radical cystectomy? BJU Int. 2004. 93:64–66.
27. Kwak JJ, Kim TH, Sung GT. Short term outcomes of laparoscopic radical cystectomy with an extracorporeal ileal conduit: comparative analysis with the open method. Korean J Urol. 2007. 48:938–944.
28. Haber GP, Gill IS. Laparoscopic radical cystectomy for cancer: oncological outcomes at up to 5 years. BJU Int. 2007. 100:137–142.
29. Abraham JB, Young JL, Box GN, Lee HJ, Deane LA, Ornstein DK. Comparative analysis of laparoscopic and robot-assisted radical cystectomy with ileal conduit urinary diversion. J Endourol. 2007. 21:1473–1480.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download