Abstract
Purpose
Low urinary pH is a risk factor for uric acid stones, and acidic urine has been described as a renal manifestation of the metabolic syndrome. We evaluated the association between metabolic syndrome and urinary pH in adult Korean men who visited a health promotion center.
Materials and Methods
From 2004 to 2008, a total of 18,513 adult men who visited our health promotion center were enrolled in this study. The relation between urinary pH and various parameters associated with the metabolic syndrome were evaluated.
Results
The average age was 45.6 years (range, 18-95 years), and 4987 men (26.9%) were classified as having the metabolic syndrome. The mean urinary pH of the metabolic syndrome group was 5.91, which was significantly lower than that of the normal group (6.08). In univariate and multivariate analysis, body mass index, serum triglyceride, and blood sugar were negatively correlated with urinary pH (p<0.05). In multivariate logistic regression analysis, obesity (body mass index ≥25 kg/m2), hypertriglyceridemia (≥150 mg/dl), high fasting glucose (≥110 mg/dl), and low high-density lipoprotein cholesterol (<45 mg/dl) were the significant factors that predicted low urinary pH (≤5.5).
Figures and Tables
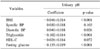
Table 3
Multivariate linear regression model between urinary pH and components of the metabolic syndrome
References
1. Borghi L, Schianchi T, Meschi T, Guerra A, Allegri F, Maggiore U, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002. 346:77–84.
2. Maalouf NM, Cameron MA, Moe OW, Sakhaee K. Novel insights into the pathogenesis of uric acid nephrolithiasis. Curr Opin Nephrol Hypertens. 2004. 13:181–189.
3. Coe FL. Uric acid and calcium oxalate nephrolithiasis. Kidney Int. 1983. 24:392–403.
4. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988. 37:1595–1607.
5. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005. 365:1415–1428.
6. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998. 97:1837–1847.
7. Abate N, Chandalia M, Cabo-Chan AV Jr, Moe OW, Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int. 2004. 65:386–392.
8. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001. 285:2486–2497.
9. Ford ES, Mokdad AH, Giles WH. Trends in waist circumference among US adults. Obes Res. 2003. 11:1223–1231.
10. Oh SW, Yoon YS, Shin SA. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol. 2005. 23:4742–4754.
11. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004. 363:157–163.
12. DuBose TD Jr, Good DW, Hamm LL, Wall SM. Ammonium transport in the kidney: New physiological concepts and their clinical implications. J Am Soc Nephrol. 1991. 1:1193–1203.
13. Wasko R, Frankenfield BA. Allopurinol dissolution of renal uric acid calculi. JAMA. 1968. 205:801.
14. Stoller ML. Gout and stones or stones and gout? J Urol. 1995. 154:1670.
15. Maalouf NM, Cameron MA, Moe OW, Adams-Huet B, Sakhaee K. Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol. 2007. 2:883–888.
16. Daudon M, Lacour B, Jungers P. High prevalence of uric acid calculi in diabetic stone formers. Nephrol Dial Transplant. 2005. 20:468–469.
17. Pak CY, Sakhaee K, Moe O, Preminger GM, Poindexter JR, Peterson RD, et al. Biochemical profile of stone-forming patients with diabetes mellitus. Urology. 2003. 61:523–527.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download