Abstract
Purpose:
Because insulin resistance may be related to prostate cancer (PCa) initiation and progression, and adipokines target preneoplastic cells, leading to activation of signaling cascades that can promote aberrant cellular proliferation and transformation to a malignant phenotype, we hypothesized that increased resistin protein in prostate epithelial cells might underlie the association between PCa and insulin resistance.
Materials and Methods:
In this study, we investigated the intensity of prostate epithelial resistin expression by immunohistochemical staining in 67 patients with PCa and 26 patients with benign prostatic hyperplasia (BPH). The body mass index (BMI) and age of the groups were similar. Patients with PCa were stratified into 3 groups according to the spread of the disease as organ-confined, locally advanced, or metastatic disease and according to the grade.
Results:
The intensity of prostate epithelial resistin expression and the mean prostate-specific antigen (PSA) were significantly greater in the PCa group than in the BPH group. Additionally, according to progression of PCa, mean PSA, mean age, and the intensity of resistin expression were significantly increased. With higher Gleason score of PCa, age and the intensity of resistin expression were significantly increased.
Go to : 
REFERENCES
1.Fair WR., Fleshner NE., Heston W. Cancer of the prostate: a nutritional disease? Urology. 1997. 50:840–8.

2.Pu YS., Chiang HS., Lin CC., Huang CY., Huang KH., Chen J. Changing trends of prostate cancer in Asia. Aging Male. 2004. 7:120–32.

3.Bosland MC., Oakley-Girvan I., Whittemore AS. Dietary fat, calories, and prostate cancer risk. J Natl Cancer Inst. 1999. 91:489–91.

4.Calle EE., Rodriguez C., Walker-Thurmond K., Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003. 348:1625–38.
5.Severson RK., Grove JS., Nomura AM., Stemmermann GN. Body mass and prostatic cancer: a prospective study. BMJ. 1988. 297:713–5.

6.Lyon CJ., Law RE., Hsueh WA. Minireview: adiposity, inflammation, and atherogenesis. Endocrinology. 2003. 144:2195–200.

7.Steppan CM., Bailey ST., Bhat S., Brown EJ., Banerjee RR., Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001. 409:307–12.

8.Holcomb IN., Kabakoff RC., Chan B., Baker TW., Gurney A., Henzel W, et al. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 2000. 19:4046–55.

9.Calabro P., Samudio I., Willerson JT., Yeh ET. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation. 2004. 110:3335–40.

10.Di Simone N., Di Nicuolo F., Sanguinetti M., Castellani R., D' Asta M., Caforio L, et al. Resistin regulates human choriocarcinoma cell invasive behaviour and endothelial cell angiogenic processes. J Endocrinol. 2006. 189:691–9.

11.Kang JH., Yu BY., Youn DS. Relationship of serum adiponectin and resistin levels with breast cancer risk. J Korean Med Sci. 2007. 22:117–21.

12.Tilg H., Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006. 6:772–83.

13.Flegal KM., Carroll MD., Ogden CL., Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002. 288:1723–7.

14.Annual Report of Korea Central Cancer Registry (Based on registered data from 139 hospitals). Korea central cancer registry, Ministry of health and welfare, Republic of Korea. 2003.
15.Mokdad AH., Ford ES., Bowman BA., Dietz WH., Vinicor F., Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003. 289:76–9.

16.Engeland A., Tretli S., Bjorge T. Height, body mass index, and prostate cancer: a follow-up of 950000 Norwegian men. Br J Cancer. 2003. 89:1237–42.

17.Freedland SJ., Terris MK., Presti JC Jr., Amling CL., Kane CJ., Trock B, et al. Obestiy and biochemical outcome following radical prostatectomy for organ confined disease with negative surgical margins. J Urol. 2004. 172:520–4.
18.Chang SS., Doung DT., Wells N., Cole EE., Smith JA Jr., Cookson MS. Predicting blood loss and transfusion requirements during radical prostatectomy: the significant negative impact of increasing body mass index. J Urol. 2004. 171:1861–5.

19.Amling CL., Rifferburgh RH., Sun L., Moul JW., Lance RS., Kusuda L, et al. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol. 2004. 22:439–45.

20.Siddiqui SA., Inman BA., Sengupta S., Slezak JM., Bergstralh EJ., Leibovich BC, et al. Obesity and survival after radical prostatectomy: a 10-year prospective cohort study. Cancer. 2006. 107:521–9.

21.Calle EE., Rodriguez C., Walker-Thurmond K., Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003. 348:1625–38.
22.Youn BS., Yu KY., Park HJ., Lee NS., Min SS., Youn MY, et al. Plasma resistin concentrations measured by enzyme-linked immunosorbent assay using a newly developed monoclonal antibody are elevated in individuals with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2004. 89:150–6.

23.Shalev A., Patterson NB., Hirshberg B., Rother KI., Harlan DM. Resistin serum levels in type 1 diabetes pre- and post-islet transplantation. Metabolism. 2004. 53:403–4.

24.Stattin P., Soderberg S., Hallmans G., Bylund A., Kaaks R., Sten-man UH, et al. Leptin is associated with increased prostate cancer risk: a nested case-referent study. J Clin Endocrinol Metab. 2001. 86:1341–5.

25.Kim JH., Lee SY., Myung SC., Kim YS., Kim TH., Kim MK. Clinical significance of the leptin and leptin receptor expressions in prostate tissues. Asian J Androl. 2008. 10:923–8.

Go to : 
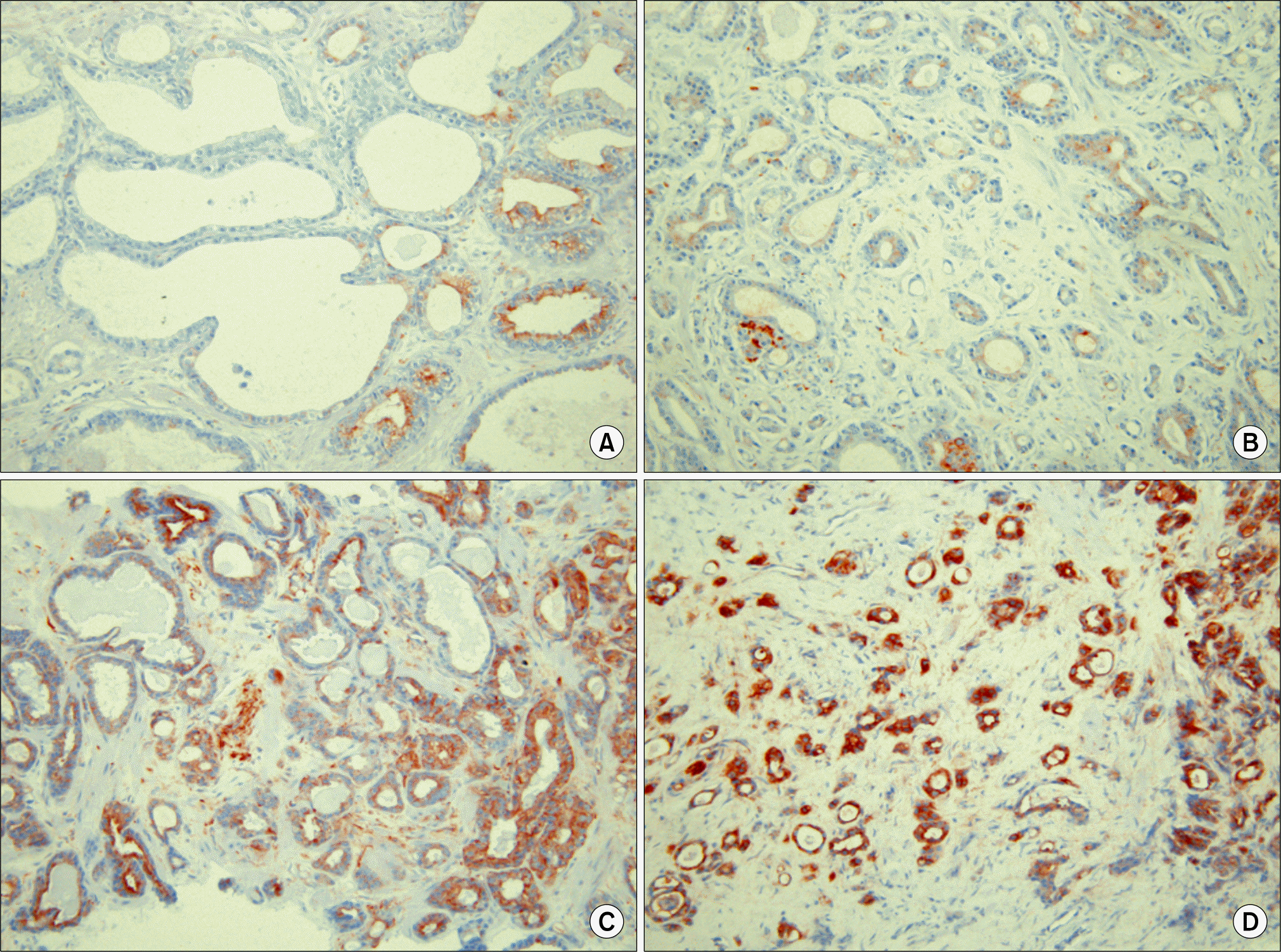 | Fig. 1Resistin immunohistochemical staining in prostate epithelial cells in (A) benign prostate hyperplasia, and in (B) low-grade, (C) intermediate-grade, and (D) high-grade of prostate cancer (PCa) specimens (anti-resistin antibody, x100 original magnification). The intensity of prostate epithelial resistin expression was significantly greater in the PCa group than in the benign group (p<0.001), and was significantly increased with higher grade (p<0.001). PCa: prostate cancer. |
Table 1.
Comparison of parameters assessed in patients in the prostate cancer (PCa) and benign prostatic hyperplasia (BPH) groups
| BPH (n=26) | PCa (n=67) | p-value | |
|---|---|---|---|
| Age (years) | 65 (52-88) | 63.5 (45-82) | 0.775a |
| BMI (kg/m2) | 24.1 (19.3-28.9) | 23.25 (19.0-27.5) | 0.832a |
| PSA (ng/ml) | 2.16 (0.29-3.94) | 1,362.13 (4.25-2,720) | <0.05a |
| Fasting glucose (mg/dl) | 127.5 (77-178) | 125 (92-158) | 0.816a |
| Total cholesterol (mg/dl) | 202.5 (154-251) | 186 (82-290) | 0.132a |
| Intensity of resistin (%) | <0.001b | ||
| 1+ | 16 (61.5) | 15 (22.4) | |
| 2+ | 9 (34.6) | 27 (40.3) | |
| 3+ | 1 (3.8) | 25 (37.3) |
Table 2.
Comparison of parameters in those with organ-confined, locally advanced, or metastatic prostate cancer (PCa)
| Organ-confined (n=37) | Locally advanced (n=13) | Metastatic (n=17) | p-value | |
|---|---|---|---|---|
| Age (years) | 65.5 (45-86) | 64.5 (53-76) | 72 (62-82) | <0.01a |
| BMI (kg/m2) | 24 (19-29) | 24.05 (21.3-26.8) | 23.8 (20.1-27.5) | 0.410a |
| PSA (ng/ml) | 45.33 (4.25-86.41) | 26.24 (4.81-47.67) | 1,360.9 (1.79-2,720) | <0.01a |
| Fasting glucose (mg/dl) | 143 (92-194) | 125.5 (79-172) | 136 (77-195) | 0.412a |
| Total cholesterol (mg/dl) | 170 (82-258) | 201 (127-275) | 223 (156-290) | 0.614a |
| Intensity of resistin (%) | <0.05b | |||
| 1+ | 10 (27.0) | 2 (15.4) | 3 (17.6) | |
| 2+ | 17 (45.9) | 7 (53.8) | 3 (17.6) | |
| 3+ | 10 (27.0) | 4 (30.8) | 11 (64.7) |
Table 3.
Comparisons of parameters of low-grade, intermediate-grade, and high-grade prostate cancer (PCa)
| Low-grade GS<7 (n=23) | Intermediate-grade GS=7 (n=24) High-grade GS>7 (n=20) | p-value | ||
|---|---|---|---|---|
| Age (years) | 66 (50-82) | 60.5 (46-76) | 74 (62-86) | <0.05a |
| BMI (kg/m2) | 25.5 (22-29) | 22.9 (19-26.8) | 23.3 (19.1-27.5) | 0.177a |
| PSA (ng/ml) | 29.98 (4.25-55.7) | 1,362.41 (4.81-2,720) | 629.63 (2.25-1,257) | 0.219a |
| Fasting glucose (mg/dl) | 143.5 (93-194) | 125.5 (79-172) | 117.5 (77-158) | 0.352a |
| Total cholesterol (mg/dl) | 162 (96-228) | 178.5 (82-275) | 210 (130-290) | 0.963a |
| Intensity of resistin (%) | <0.001b | |||
| 1+ | 10 (45.3) | 4 (16.7) | 1 (5.0) | |
| 2+ | 9 (39.1) | 13 (54.2) | 5 (25.0) | |
| 3+ | 4 (17.4) | 7 (29.2) | 14 (70.0) | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download