Abstract
Purpose
Ureteropelvic junction (UPJ) obstruction is by far the most common cause of pediatric hydronephrosis. The widespread use of antenatal ultrasonography and modern imaging techniques has resulted in earlier and more common diagnosis of hydronephrosis. However, compared with this increased earlier detection, little has changed regarding the management of hydronephrosis. Through this review, we wish to provide an overview of the studies done to date and search for areas that warrant further study.
Materials and Methods
Through PubMed, we reviewed the literature on the subject of UPJ obstruction in the pediatric population. We also present data from our institution regarding recent trends in the evaluation and treatment of UPJ obstructions.
Results
In addition to conventional imaging studies, attempts are being made at making use of biochemical parameters (e.g., β2 m, N-acetyl-β-D-glucosaminidase [NAG], transforming growth factor [TGF]-β, etc.) as not only indicators of intervention but also prognostic factors during follow-up. Although we routinely use radionuclide imaging to evaluate renal function, a more accurate novel tool that can represent true renal function is needed. With the development in the field of laparoscopic and minimally invasive surgery, the role of laparoscopy and robot-assisted laparoscopic pyeloplasty is expanding, even in the pediatric population. However, relatively little is known about the factors that might be associated with postoperative outcomes.
Ureteropelvic junction (UPJ) obstruction is by far the most common cause of pediatric hydronephrosis, occurring in 1 per 1,000-2,000 newborns. Widespread use of antenatal ultrasonography and the advent of modern imaging techniques have resulted in earlier and more common diagnosis of hydronephrosis.1,2
Obstruction is more commonly found in boys than in girls, especially in the newborn period, when the ratio exceeds 2:1. Left-sided lesions predominate, particularly in the neonate, up to approximately 67%, and bilateral cases are observed in 10-40% of cases; however, fewer than 5% of patients require bilateral repair.3
With more early-detected cases, the management of pediatric UPJ obstruction remains more important. Choosing an optimal therapeutic regimen is difficult due to the high variability in function, degree of obstruction, extent of damage, and potential for regeneration in growing kidneys.
In the embryogenesis, the UPJ is formed during the fifth week. By weeks 10-12 of gestation, the initial tubular lumen of the ureteric bud becomes recanalized, and the UPJ area is the last to recanalize. Inadequate canalization of this area is the main embryological explanation of UPJ obstruction.4 Several growth factors may control embryogenesis of UPJ. Researchers propose that improper innervation with diminished synaptic vesicles may be a factor in the development of UPJ obstruction, and factors involved in neuronal development, such as protein gene product (PGP) 9.5 (a general neuronal marker), S-100 protein (a nerve supporting cell marker), synaptophysin (a synapse vesical marker), and nerve growth factor receptor were all decreased in the resected specimens of UPJ.
The induction of kidney mesenchyme by the ureteric bud is mediated by a transcription factor Pax-2.5 Other factors, such as c-ret, kdn-1, and wt1, also may be involved. A well-known growth factor, transforming growth factor (TGF), may account for the abnormal smooth musculature in the obstructed renal pelvis.6 More research certainly is needed to clarify the molecular basis of the UPJ obstruction. This intrinsic obstruction is evident as the ureteral narrowing with angulation is found. During exploration, a catheter usually is passed to the renal pelvis without resistance, and this is evidence of the fact that the true narrowing is not a main pathologic change in UPJ obstruction. Some claimed the presence of remnant valvular mucosal folds, while others postulate the disproportionate abundance of longitudinal muscles as the cause of this condition.7
The most attractive theory is that the obstruction is secondary to muscular discontinuity, which disrupts the coordinated motion of smooth muscle cells and may result in impeded transport of urine and blockage of the downward transmission of ureteral peristalsis.8 This absence or disorientation of smooth muscle fibers at UPJ is clearly evident on electron microscope evaluation with the findings of hypotrophy/hypertrophy of the smooth muscle and its replacement with excessive collagen, combined with diminution of nerve terminals and nerves at the stenotic portion.9
One study had identified altered expression of interstitial Cajal cells in obstructed UPJ specimens, which are normally intercalated between nerve terminal and smooth muscle cells, providing a means of transducing signals from neurotransmitters and mediating neurotransmission.10 This suggests that UPJO may cause the failure of transmission of peristaltic waves across the UPJ, resulting in the failure of urine to be propelled from the renal pelvis into the ureter.
Extrinsic obstructions secondary to bands, kinks, and aberrant vessels also are commonly encountered. In 40% of cases, an aberrant, accessory, or early-branching lower pole segment vessel is found and observed to compress the ureter, causing mechanical obstruction. In this case, with the increased urine volume, the UPJ angulation with intrapelvic volume expansion causes increased resistance and obstruction.11 Further angulation may occur as it becomes adherent to an inflammatory process. The presence of such a vessel in the vicinity of UPJ has gained recent attention after the advent of the endourological management. The anterior surface of the renal pelvis is associated with a lower pole vessel in 65% of cases, whereas the posterior surface is in contact with a vessel in 6% of the kidneys examined.12 This information is relevant for the endoscopic incision of UPJ, making lateral incision the only safe option.
Patients with extrinsic obstructions present rather late in childhood, with intermittent abdominal or flank pain.13 Horseshoe or pelvic kidney, duplex collecting systems, and other rotational abnormalities also may cause UPJ obstruction.14 Cases of so-called high inserted ureter-to-renal pelvis exist, but this is presumed to be a secondary phenomenon to obstruction because the ureteral insertion seems to be higher in cases of dilated renal pelvis.
The urinary drainage from renal pelvis to ureter is determined by many factors. Urine volume and flow, the degree of UPJ obstruction, the functional capacity of glomerulus and collecting system, and the compliance of renal pelvis are the 4 main variables determining the pelvic pressure. At first, in response to the increased pelvic pressure, the renal pelvis dilates and ureteral muscles show hypertrophy.15 In the intrarenal type of obstruction, the degree of dilation is restricted by renal parenchyme; thus, the damage usually is more severe than the extrarenal type. Parenchymal damage by UPJ obstruction is well documented by histologic changes, which are more severe in cases of differential function of less than 35%.16
Experimental studies using artificially made complete obstructions showed changes that suggest the upward transmission of ureteral pressure in an obstructed kidney and the subsequent effects on tubular pressure, tubular function, renal blood flow (RBF), and glomerular filtration rate (GFR).17-20 The urinary obstruction results in the impairment of all renal functions except urinary dilution; however, the elevation of ureteral pressure above a certain point had no further effect on intratubular pressure. With complete ureteral ligation, a rise in renal pelvic pressure occurs, which is only transitory; over a period of hours, the renal pelvic pressure falls in concert with RBF. Also, the intrapelvic pressure in patients with UPJ obstruction most often is in the normal range assessed at the time of surgery. Researchers propose that the expansion of the renal pelvis is protective by dampening out of the pressure.
Koff proposed the concept of pressure- or volume-dependent flow.21,22 In instances of intrinsic obstruction, at low urinary flow rates, no obstruction exists; however, as the flow rate increases, the urinary bolus are not conducted, causing the renal pelvis to distend. This concept is called a pressure-dependent flow pattern. On the contrary, in cases of extrinsic compression usually caused by aberrant vessels, urine flow is impeded only after a definite amount of urine is collected in the renal pelvis. This is an example of volume-dependent flow, and the pressure damage is only evident intermittently; thus, the degree of damage generally is less than that of intrinsic obstruction.
Significant urinary obstruction invariably results in tubular dilation, glomerulosclerosis, inflammation, and fibrosis,23 not only in the affected kidney, but also in the contralateral kidney.24 Although not absolute, a good correlation exists between the severity of these histologic changes and the function remaining in the affected kidneys. Sclerotic glomeruli and fibrosis are reliably localized to areas of the kidney that demonstrate the most inflammatory infiltrate. The infiltrate consists mostly of mononuclear cells in both the cortex and medulla.25 The cells predominantly are macrophages, though a small number of T cells are present.26
The activation of the renin-angiotensin system is a major factor in partial obstruction.27 Administration of the angiotensin-converting enzyme (ACE) inhibitor enalapril not only maintained RBF in partially obstructed kidneys at 3 weeks post-obstruction but also prevented the histologic changes of glomerulosclerosis.28 The effects of obstruction are not all ischemic. Obstruction can mimic renal artery stenosis, and, because of its intense vasoconstrictor action, the resulting increase in angiotensin II (AII) leads to decreases in GFR. It is becoming increasingly clear, however, that AII profoundly affects the expression of growth factors in the developing kidney that ultimately are responsible for the changes in the histology. Up regulation of TGF is apparent in these infiltrating cells, and the degree of up regulation correlates directly with fibrosis and collagen deposition in obstructed kidneys.29
Novel approaches may discern the clinically significant UPJ obstruction.
Disruption of proximal tubular integrity leads to increased urinary concentrations of β2-microglobulin (B2M), which normally is resorbed from the tubular lumen via phagocytosis and lysosomal digestion.30
Functionally significant obstruction and recovery from obstruction may be determined by following the urinary concentration of B2M.31 However, many different insults other than UPJ obstruction can lead to increased levels of B2M in the urine.
NAG is a tubular lysosomal enzyme present in the urine of children who have various renal diseases.
In rats with experimental partial ureteral obstruction, the urinary concentration of NAG increases in the first 2 weeks of obstruction and decreases with the relief of obstruction.32 In a clinical study, urinary NAG levels in kidneys at the time of pyeloplasty were 7 fold higher than in bladder compared to normal controls. In addition, enzyme levels in the bladder of patients 6 weeks after surgery suggested normalization of NAG excretion.32
During any session of prenatal ultrasonographic diagnosis, thoroughly investigate the following from the initial study usually performed between 16 and 20 weeks: amniotic fluid volume to rule out oligohydramnios, bladder volume, kidney size, anteroposterior diameter of the renal pelvis, and any associated abnormalities. Following fetal hydronephrosis also is important to monitor possible progression. A meta-analysis of 7 studies on isolated antenatal hydronephrosis showed that 98% of patients with Society of Fetal Urology (SFU) grades33 1-2 hydronephrosis (anterior-posterior pelvic diameter [APPD] <12 mm) resolved, stabilized, or improved during follow-up.
After the prenatal presumptive diagnosis UPJ obstruction or other conditions causing hydronephrosis is made, the neonate should undergo ultrasonographic evaluation on the second or third day of life. Before this date, results may be false negative because of neonatal dehydration and physiologic oliguria; however, in cases of bilateral hydronephrosis, more rapid evaluation is warranted. Postnatal examination evaluation consists of urinary tract study whether the calyceal pelvic dilation with or without renal cortical thinning is present. Approximately 20% of antenatal hydronephrosis are not found on postnatal ultrasonogram.34 Doppler sonography is especially reliable in the preoperative diagnosis of aberrant-accessory blood vessels associated with UPJ obstruction.35,36 At the same time, ultrasonographic evaluation on the contralateral kidney, bladder, and ureter is performed.
The renal scan and scintigraphy (ie, diuretic renogram) is the most widely used technique in the presence of hydronephrosis to assess function and obstruction.37 The rate at which tracer leaves the renal pelvis following diuretic injection, reflected in the slope of the drainage curve and often reported as T1/2 (the time required for 50% of the isotope to exit), is generally viewed as a reflection of the patency of the UPJ. Rapid drainage indicates no obstruction, while impaired drainage or slow or no washout (T1/2 >20 min) indicates obstruction.
The current radiopharmaceutical agent most widely used is technetium 99m diethylenetriamine pentaacetic acid (99mTc-DTPA). It is excreted by glomerular filtration and is not secreted or reabsorbed by the renal tubules.38 Another agent is 99mTc-mercaptoacetyltriglyine (MAG3), which offers better anatomical resolution and can be used in case of decreased renal function. Variables include the use of intravenous hydration, the dosage and timing of administration of diuretic, the requirement for bladder catheterization, the degree of pelvic dilatation, the severity of outflow obstruction, and the method of calculating the clearance after the administration of diuretic.39,40 In order to overcome such variables, Conway and Maizels suggested the use of a "well tempered" diuretic renogram in neonates with hydronephrosis.41
The most useful measure in diuretic renography is the estimate of differential renal function. This is considered significant when it is less than 40%. This percentage usually is well correlated with the half-life (T1/2) washout curve. As stated above, many factors must be considered when evaluating the renal scan, especially in neonates (Table 1). For this reason, the T1/2 of the diuretic renogram cannot be a single indicator to determine surgery, especially in the neonate.
Although it is postulated that significant obstruction results in decreased ipsilateral renal function, we often see maintained or even increased differential renal function on a renal scan of a large hydronephrotic kidneys. This paradoxically increased renal function, the so-called supranormal renal function, is usually defined as a differential renal function greater than 55%, given that the contralateral kidney and bladder function are normal.42 It has been hypothesized to be caused by an increase in single nephron filtration or nephron volume.42 However, in a study of histopathological changes of hydronephrotic kidneys with supranormal DRF assessed with intraoperative kidney biopsy at the time of pyeloplasty, the glomerular area was not significantly larger than controls, but the probability for a larger renal glomeruli increased with decreasing DRF.43 Instead of increased nephron volume, the supranormal DRF can be accounted for by an increase in renal blood flow that results from tubuloglomerular feedback, prostaglandins, and the renin-angiotensin system as a protective mechanism from high intrapelvic pressure.
The timing of surgical correction of hydronephrosis suggestive of UPJ obstruction in newborns is highly controversial. Those who support delayed management contend that for most newborns with relatively preserved differential renal function (>35% of differential renal function), hydronephrosis is a relatively benign disease without proof of progression.44,45 Renal function does not deteriorate; thus, immediate surgery is not necessary. In a study by Koff and associates,46 approximately 81 out of 104 patients were followed for 5 years; 7 (7%) of those patients ultimately required pyeloplasty, and, even in these cases, pyeloplasty successfully restored the differential renal function to pre-deterioration levels. Onen et al. suggested that nonoperative management with close follow up during the first 2 years appears to be a safe approach even in neonates with severe bilateral UPJ obstruction.47
Researchers also observed that in 15 out of 16 patients with severe hydronephrosis (grade 4 hydronephrosis according to the SFU Guidelines) associated with a differential renal function of less than 40%, spontaneous improvement occurred in the initial obstructive patterns on renal scans, and, for 6 of the patients, it became non-obstructed. Similar results were observed with ultrasonography that hydronephrosis disappeared in 6 kidneys and improved in another 6 kidneys.
Thus, the difficulty in determining the indication of surgical management is 2-fold. First, ultrasonography and diuretic renography to assess hydronephrosis are inaccurate and sometimes misleading. Second, some significant cases of hydronephrosis that are discovered by these modalities may not be obstructive at all. Therefore, Koff redefined obstruction as "any restriction to urinary outflow, which if untreated will injure the kidney" to provide a clinically useful guideline; Koff recommended that most unilateral hydronephrosis actually is non-obstructed and, thus, benign, which can be observed safely nonoperatively.48
Physiologically, the newborn kidney is quite different than the adult kidney, particularly in the response to stimulation by the renin-angiotensin system. Renal function could be preserved by relieving the obstruction, achieving maximal benefit in the youngest of kidneys.49,50
Unilateral pyeloplasty not only improves hydronephrosis but also significantly increases creatinine clearance (as calculated by the Schwartz formula) and somatic growth.51 The implication is that unilateral obstruction has negative effects on renal function and on somatic growth. Spontaneous resolution of hydronephrosis is not as benign as proposed by Koff and Campbell, that is 15-33% of patients with asymptomatic neonatal hydronephrosis show progressive ipsilateral renal deterioration, and about one half of them never regain the lost function by pyeloplasty.52
There are 2 well-designed longitudinal studies that provide valuable information against initial observation and delayed management. According to the study by Ransley53 and associates, of 100 infants with DRF of hydronephrotic kidney higher than 40% who were followed non-operatively, 23 eventually underwent pyeloplasty during 6 years of follow-up care. Among these patients, 5 (36%) completely recovered renal function, 4 (29%) partially recovered, 3 (21%) had no change, and 1 (7%) further deteriorated after pyeloplasty. Thus, 8 of 100 initially well-functioning kidneys sustained permanent deterioration with this approach. Similar results were observed by Cartwright and Duckett,54 which included the results with 39 infants with a cut-off value of 35% of differential renal function. Six patients (15%) underwent pyeloplasty because of decreasing renal function, UTI, or pain.
A differential renal function below 40% in unilateral hydronephrosis with a normal contralateral kidney, recurrent urinary tract infections in spite of antibiotic prophylaxis and rapid aggravation of hydronephrosis on serial renal ultrasound or severe bilateral hydronephrosis seem to be universally recognized as indications for surgical intervention.55
The technique of complete ureteral transection followed by reanastomosis to the renal pelvis was first described in the management of a retrocaval ureter, but it was easily adapted for reconstructing the UPJ obstruction. Many different approaches have been tried, such as lumbotomy, flank, or anterior extraperitoneal incision, but the essence of repair consists of excision of the narrowed segment, spatulation, and anastomosis to the most dependent portion of the renal pelvis. Foley YV-plasty, a non-dismembered type of repair, is useful in the repair of a kidney with high ureteral insertion and most cases of horseshoe kidneys; however, the Anderson-Hynes pyeloplasty, the most commonly used type of repair, has a high success rate with few complications in most cases.56,57
Endourologic methods applied on UPJ obstruction include balloon dilatations, percutaneous antegrade endopyelotomy, and retrograde ureteroscopic endopyelotomy. In 1983, Wickham and Kellet established access to a hydronephrotic kidney and performed the first percutaneous pyelolysis.58 Soon, a large series of endopyelotomies in adults was reported with fairly good short- and long-term success rates of 70-85%. If an initial attempt of endopyelotomy fails, subsequent open pyeloplasty is still a viable option with a high success rate.
Laparoscopic dismembered pyeloplasty, first introduced in 1993 by Schuessler,59 yields results that are comparable with those of open pyeloplasty, with success rates reported to be as high as 96-98% while still maintaining the benefits of endoscopic approaches, including less postoperative pain, short hospitalization, and reduced postoperative recovery time. Nowadays, despite the disadvantage of a limited working space, retroperitoneal laparoscopic pyeloplasties are becoming popular since unnecessary handling of the bowel can be avoided. However, the technical skills required for intracorporeal suturing and the lengthy operation time due to the degree of difficulty make it a costly procedure. The introduction of robot-assisted techniques have greatly facilitated the suturing and has become an attractive treatment option but the high cost still remains an obstacle toward the popularization of this technique.
The overall success rate with the dismembered repair is quite satisfactory; most series report a success rate of higher than 90-95%. Long-term obstruction at the anastomosis can occur; but reoperation rate for this is low, occurring in 2-5% of cases.
Bleeding and infection are uncommon following pyeloplasty. Of those with hydronephrosis and preserved renal function at neonatal evaluation, 23% presented for delayed surgery in one series. On the other hand, some propose delaying surgical correction because newborn hydronephrosis is a relatively benign condition and a definite proportion of patients have spontaneous improvement.
The common early complications are prolonged urinary extravasation and delayed opening of the anastomosis. Urinary extravasation usually stops spontaneously, generally within 2 weeks. Delay in opening of the anastomosis is observed most often with the use of a nephrostomy tube without a stent across the anastomosis. Within 3 months of surgery, 80% of obstructed anastomosis eventually open. According to our review, significant improvements in hydronephrosis could be observed until 6 months postoperatively, but no significant changed occurred beyond 6 months.60
Numerous prognostic factors have been investigated to predict the postoperative outcome. In a review of 30 patients who underwent pyeloplasty for unilateral UPJ onstruction by Park et al, they found no significant difference in the age, sex, history of UTI, anteroposterior diameter of renal pelvis, parenchymal thickness or laterality between the group which showed improvement and aggravation following pyeloplasty.61
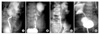
The value of visualizing the ureter and the clinical significance of the ureter morphology has been suggested by several studies. Cockrell reported that 36% of his patients had "more than a simple narrowing" in the UPJ, and suggested visualization of ureters could contribute to successful surgery.62 In our study of intraoperative retrograde pyelograms, patients with hypoplastic ureters tended to show a slower improvement rate compared to other types of ureteral narrowing (Fig. 1).63
Patients with lower percentages of elastin in the renal pelvis, UPJ proper, or ureter tended to show better resolution of hydronephrosis 6 months after pyeloplasty. Increased elastin of the renal pelvis and ureter might result in inelasticity and low compliance, which delays hydronephrosis improvement after pyeloplasty.64
The meaning of supranormal function and its implication in postoperative renal function also warrants further investigation. Although some authors have suggested that the supranormal function reprsents a true renal function of the affected kidney and remains supranormal even after pyeloplasty in most cases,65 it is debatable whether supranormal function can be considered as a favorable prognostic factor and longer follow up is needed.
In spite of the vast number of reports and research performed, ureteropelvic junction obstruction has not been completely uncovered. Finding an accurate, yet easily applicable method of evaluating the true renal function might bring along major changes both in the diagnosis and treatment of the disease.
Figures and Tables
References
1. Capello SA, Kogan BA, Giorgi LJ Jr, Kaufman RP Jr. Prenatal ultrasound has led to earlier detection and repair of ureteropelvic junction obstruction. J Urol. 2005. 174:1425–1428.
2. Wiener JS, Emmert GK, Mesrobian HG, Whitehurst AW, Smith LR, King LR. Are modern imaging techniques over diagnosing ureteropelvic junction obstruction? J Urol. 1995. 154:659–661.
3. Carr MC, El-Ghoneimi A. Campbell MF, Wein AJ, Kavoussi LR, editors. Anomalies and surgery of the ureteropelvic junction in children. Campbell-Walsh urology. 2007. 9th ed. Philadelphia: Saunders Elsevier;3359.
4. Ruano-Gil D, Coca-Payeras A, Tejedo-Mateu A. Obstruction and normal recanalization of the ureter in the human embryo. Its relation to congenital ureteric obstruction. Eur Urol. 1975. 1:287–293.
5. Dressler GR, Wilkinson JE, Rothenpieler UW, Patterson LT, Williams-Simons L, Westphal H. Deregulation of Pax-2 expression in transgenic mice generates severe kidney abnormalities. Nature. 1993. 362:65–67.
6. Furness PD 3rd, Maizels M, Han SW, Cohn RA, Cheng EY. Elevated bladder urine concentration of transforming growth factor-beta1 correlates with upper urinary tract obstruction in children. J Urol. 1999. 162:1033–1036.
7. Maizels M, Stephens FD. Valves of the ureter as a cause of primary obstruction of the ureter: anatomic, embryologic and clinical aspects. J Urol. 1980. 123:742–747.
8. Murnaghan GF. The dynamics of the renal pelvis and ureter with reference to congenital hydronephrosis. Br J Urol. 1958. 30:321–329.
9. Hosgor M, Karaca I, Ulukus C, Ozer E, Ozkara E, Sam B, et al. Structural changes of smooth muscle in congenital ureteropelvic junction obstruction. J Pediatr Surg. 2005. 40:1632–1636.
10. Solari V, Piotrowska AP, Puri P. Altered expression of interstitial cells of Cajal in congenital ureteropelvic junction obstruction. J Urol. 2003. 170:2420–2422.
11. Stephens FD. Ureterovascular hydronephrosis and the "aberrant" renal vessels. J Urol. 1982. 128:984–987.
12. Sampaio FJ. Vascular anatomy at the ureteropelvic junction. Urol Clin North Am. 1998. 25:251–258.
13. Ross JH, Kay R, Knipper NS, Streem SB. The absence of crossing vessels in association with ureteropelvic junction obstruction detected by prenatal ultrasonography. J Urol. 1998. 160:973–975.
14. Ross JH, Kay R. Ureteropelvic junction obstruction in anomalous kidneys. Urol Clin North Am. 1998. 25:219–225.
15. Klahr S. Pathophysiology of obstructive nephropathy. Kidney Int. 1983. 23:414–426.
16. Stock JA, Krous HF, Heffernan J, Packer M, Kaplan GW. Correlation of renal biopsy and radionuclide renal scan differential function in patients with unilateral ureteropelvic junction obstruction. J Urol. 1995. 154:716–718.
17. Josephson S. Experimental obstructive hydronephrosis in newborn rats. III. Long-term effects on renal function. J Urol. 1983. 129:396–400.
18. Josephson S, Ericson AC, Sjoquist M. Experimental obstructive hydronephrosis in newborn rats. VI. Long-term effects on glomerular filtration and distribution. J Urol. 1985. 134:391–395.
19. Josephson S, Robertson B, Claesson G, Wikstad I. Experimental obstructive hydronephrosis in newborn rats. I. Surgical technique and long-term morphologic effects. Invest Urol. 1980. 17:478–483.
20. Josephson S, Wolgast M, Ojteg G. Experimental obstructive hydronephrosis in newborn rats. II. Long-term effects on renal blood flow distribution. Scand J Urol Nephrol. 1982. 16:179–185.
21. Koff SA. Pathophysiology of ureteropelvic junction obstruction. Clinical and experimental observations. Urol Clin North Am. 1990. 17:263–272.
22. Koff SA, Hayden LJ, Cirulli C, Shore R. Pathophysiology of ureteropelvic junction obstruction: experimental and clinical observations. J Urol. 1986. 136:336–338.
23. Steinhardt GF, Ramon G, Salinas-Madrigal L. Glomerulosclerosis in obstructive uropathy. J Urol. 1988. 140:1316–1318.
24. Ekinci S, Ciftci AO, Atilla P, Muftuoglu S, Senocak ME, Buyukpamukcu N. Ureteropelvic junction obstruction causes histologic alterations in contralateral kidney. J Pediatr Surg. 2003. 38:1650–1655.
25. Chiou YY, Shieh CC, Cheng HL, Tang MJ. Intrinsic expression of Th2 cytokines in urothelium of congenital ureteropelvic junction obstruction. Kidney Int. 2005. 67:638–646.
26. Schreiner GF, Harris KP, Purkerson ML, Klahr S. Immunological aspects of acute ureteral obstruction: immune cell infiltrate in the kidney. Kidney Int. 1988. 34:487–493.
27. Yarger WE, Schocken DD, Harris RH. Obstructive nephropathy in the rat: possible roles for the renin-angiotensin system, prostaglandins, and thromboxanes in postobstructive renal function. J Clin Invest. 1980. 65:400–412.
28. Ishidoya S, Morrissey J, McCracken R, Klahr S. Delayed treatment with enalapril halts tubulointerstitial fibrosis in rats with obstructive nephropathy. Kidney Int. 1996. 49:1110–1119.
29. Pimentel JL Jr, Sundell CL, Wang S, Kopp JB, Montero A, Martinez-Maldonado M. Role of angiotensin II in the expression and regulation of transforming growth factor-beta in obstructive nephropathy. Kidney Int. 1995. 48:1233–1246.
30. Tataranni G, Farinelli R, Zavagli G, Logallo G, Farinelli A. Tubule recovery after obstructive nephropathy relief: the value of enzymuria and microproteinuria. J Urol. 1987. 138:24–27.
31. Zanardo V, Da Riol R, Faggian D, Plebani M, Largajolli G, Zacchello G. Urinary beta-2-microglobulin excretion in prematures with respiratory distress syndrome. Child Nephrol Urol. 1990. 10:135–138.
32. Huland H, Gonnermann D, Werner B, Possin U. A new test to predict reversibility of hydronephrotic atrophy after stable partial unilateral ureteral obstruction. J Urol. 1988. 140:1591–1594.
33. Fernbach SK, Maizels M, Conway JJ. Ultrasound grading of hydronephrosis: introduction to the system used by the Society for Fetal Urology. Pediatr Radiol. 1993. 23:478–480.
34. Dejter SW Jr, Gibbons MD. The fate of infant kidneys with fetal hydronephrosis but initially normal postnatal sonography. J Urol. 1989. 142:661–662.
35. Mearini L, Rosi P, Zucchi A, Del Zingaro M, Mearini E, Costantini E. Color Doppler ultrasonography in the diagnosis of vascular abnormalities associated with ureteropelvic junction obstruction. J Endourol. 2003. 17:745–750.
36. Kincaid W, Hollman AS, Azmy AF. Doppler ultrasound in pelviureteric junction obstruction in infants and children. J Pediatr Surg. 1994. 29:765–768.
37. Kass EJ, Fink-Bennett D. Contemporary techniques for the radioisotopic evaluation of the dilated urinary tract. Urol Clin North Am. 1990. 17:273–289.
38. Heyman S, Duckett JW. The extraction factor: an estimate of single kidney function in children during routine radionuclide renography with 99mtechnetium diethylenetriaminepentaacetic acid. J Urol. 1988. 140:780–783.
39. Roarke MC, Sandler CM. Provocative imaging. Diuretic renography. Urol Clin North Am. 1998. 25:227–249.
40. Koff SA, Binkovitz L, Coley B, Jayanthi VR. Renal pelvis volume during diuresis in children with hydronephrosis: implications for diagnosing obstruction with diuretic renography. J Urol. 2005. 174:303–307.
41. Conway JJ, Maizels M. The "well tempered" diuretic renogram: a standard method to examine the asymptomatic neonate with hydronephrosis or hydroureteronephrosis. A report from combined meetings of The Society for Fetal Urology and members of The Pediatric Nuclear Medicine Council--The Society of Nuclear Medicine. J Nucl Med. 1992. 33:2047–2051.
42. Capolicchio G, Jednak R, Dinh L, Salle JL, Brzezinski A, Houle AM. Supranormal renographic differential renal function in congenital hydronephrosis: fact, not artifact. J Urol. 1999. 161:1290–1294.
43. Ham WS, Jeong HJ, Han SW, Kim JH, Kim DK. Increased nephron volume is not a cause of supranormal renographic differential renal function in patients with ureteropelvic junction obstruction. J Urol. 2004. 172:1108–1110.
44. Dejter SW Jr, Eggli DF, Gibbons MD. Delayed management of neonatal hydronephrosis. J Urol. 1988. 140:1305–1309.
45. Ulman I, Jayanthi VR, Koff SA. The long-term followup of newborns with severe unilateral hydronephrosis initially treated nonoperatively. J Urol. 2000. 164:1101–1105.
46. Koff SA, Campbell KD. The nonoperative management of unilateral neonatal hydronephrosis: natural history of poorly functioning kidneys. J Urol. 1994. 152:593–595.
47. Onen A, Jayanthi VR, Koff SA. Long-term followup of prenatally detected severe bilateral newborn hydronephrosis initially managed nonoperatively. J Urol. 2002. 168:1118–1120.
48. Koff SA, Campbell K. Nonoperative management of unilateral neonatal hydronephrosis. J Urol. 1992. 148:525–531.
49. Murphy JP, Holder TM, Ashcraft KW, Sharp RJ, Goodwin CD, Amoury RA. Ureteropelvic junction obstruction in the newborn. J Pediatr Surg. 1984. 19:642–648.
50. King LR, Coughlin PW, Bloch EC, Bowie JD, Ansong K, Hanna MK. The case for immediate pyeloplasty in the neonate with ureteropelvic junction obstruction. J Urol. 1984. 132:725–728.
51. Tapia J, Gonzalez R. Pyeloplasty improves renal function and somatic growth in children with ureteropelvic junction obstruction. J Urol. 1995. 154:218–222.
52. Glick PL, Harrison MR, Noall RA, Villa RL. Correction of congenital hydronephrosis in utero III. Early mid-trimester ureteral obstruction produces renal dysplasia. J Pediatr Surg. 1983. 18:681–687.
53. Ransley PG, Dhillon HK, Gordon I, Duffy PG, Dillon MJ, Barratt TM. The postnatal management of hydronephrosis diagnosed by prenatal ultrasound. J Urol. 1990. 144:584–587.
54. Cartwright PC, Duckett JW, Keating MA, Snyder HM 3rd, Escala J, Blyth B, et al. Managing apparent ureteropelvic junction obstruction in the newborn. J Urol. 1992. 148:1224–1228.
55. Chertin B, Pollack A, Koulikov D, Rabinowitz R, Hain D, Hadas-Halpren I, et al. Conservative treatment of ureteropelvic junction obstruction in children with antenatal diagnosis of hydronephrosis: lessons learned after 16 years of follow-up. Eur Urol. 2006. 49:734–738.
56. Anderson JC, Hynes W. Retrocaval ureter; a case diagnosed pre-operatively and treated successfully by a plastic operation. Br J Urol. 1949. 21:209–214.
57. Streem SB. Ureteropelvic junction obstruction. Open operative intervention. Urol Clin North Am. 1998. 25:331–341.
58. Ramsay JW, Miller RA, Kellett MJ, Blackford HN, Wickham JE, Whitfield HN. Percutaneous pyelolysis: indications, complications and results. Br J Urol. 1984. 56:586–588.
59. Schuessler WW, Grune MT, Tecuanhuey LV, Preminger GM. Laparoscopic dismembered pyeloplasty. J Urol. 1993. 150:1795–1799.
60. Park SJ, Kim YS, Lee HY, Han SW. Appropriate follow-up ultrasonography interval after pyeloplasty in children with ureteropelvic junction obstruction. Korean J Urol. 2008. 49:1018–1023.
61. Park SC, Ji YH, Park YS, Kim KS. Change of hydronephrosis after pyeloplasty in children with unilateral ureteropelvic junction obstruction. Korean J Urol. 2005. 46:586–592.
62. Cockrell SN, Hendren WH. The importance of visualizing the ureter before performing a pyeloplasty. J Urol. 1990. 144:588–592.
63. Kim JW, Han SW, Choi SK. The postoperative prognosis of ureteropelvic junction obstruction according to the appearance of the ureter of preoperative retrograde pyelography. Korean J Urol. 2003. 44:550–555.
64. Kim DS, Noh JY, Jeong HJ, Kim MJ, Jeon HJ, Han SW. Elastin content of the renal pelvis and ureter determines post-pyeloplasty recovery. J Urol. 2005. 173:962–966.
65. Song C, Park H, Park S, Moon KH, Kim KS. The change in renal function in the supranormal hydronephrotic kidney after pyeloplasty. BJU Int. 2007. 99:1483–1486.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download