Abstract
Purpose
Our study was undertaken to investigate changes in the bladder according to duration of diabetes mellitus in the Otsuka Long Evans Tokushima Fatty (OLETF) rat model, which is similar to type 2 diabetes.
Materials and Methods
OLETF rats (n=14) and Long Evans Tokushima Otsuka (LETO, n=14) rats were used. LETO is a normal control of OLETF. The animals were assigned to 4 groups: L-40 group, LETO rats 40 weeks after birth (n=7); O-40 group, OLETF rats 40 weeks after birth (n=7); L-60 group, LETO rats 60 weeks after birth (n=7); and O-60 group, OLETF rats 60 weeks after birth (n=7). At 40 weeks or 60 weeks after birth, blood glucose, cystometry, bladder weight, detrusor contractility, and mRNA expression of nerve growth factor (NGF) were assessed.
Results
Cystometry showed that diabetic bladders had increased compliance compared with the control groups at 40 and 60 weeks, and the O-60 group had greater compliance than the O-40 group. Contractile responses to electrical stimulation, bethanecol (250µM), and ATP (10 mM) were decreased in the experimental groups compared with the control groups at 40 and 60 weeks, and the O-60 group had a lower contractile response than the O-40 group. The mRNA expression of NGF was decreased in the experimental groups compared with the control groups, and the O-60 group had lower expression than the O-40 group. Changes in NGF were identified through immunohistochemical staining.
Figures and Tables
 | Fig. 1Comparison of blood glucose level in Long Evans Tokushima Otsuka (LETO) and Otsuka Long Evans Tokushima Fatty (OLETF) rats. Blood glucose levels of the experimental groups were increased compared with the control groups at 40 and 60 weeks, and the O-60 group had a higher level than the O-40 group. The data are expressed as mean±SEM. L-40 group: LETO rats 40 weeks after birth (n=7), O-40 group: OLETF rats 40 weeks after birth (n=7), L-60 group: LETO rats 60 weeks after birth (n=7), O-60 group: OLETF rats 60 weeks after birth (n=7). a: significantly different from the control groups (p<0.05), b: significantly different from the O-40 group (p<0.05). |
 | Fig. 2Comparison of bladder compliance in Long Evans Tokushima Otsuka (LETO) and Otsuka Long Evans Tokushima Fatty (OLETF) rats. The diabetic bladders had increased compliance compared with the control groups at 40 and 60 weeks, and the O-60 group had greater compliance than the O-40 group. The data are expressed as mean±SEM. L-40 group: LETO rats 40 weeks after birth (n=7), O-40 group: OLETF rats 40 weeks after birth (n=7), L-60 group: LETO rats 60 weeks after birth (n=7), O-60 group: OLETF rats 60 weeks after birth (n=7). a: significantly different from the control groups (p<0.05), b: significantly different from the O-40 group (p<0.05). |
 | Fig. 3Contractile response of the bladder muscle strip to 2, 8, 16, and 32 Hz electrical stimulation in Long Evans Tokushima Otsuka (LETO) and Otsuka Long Evans Tokushima Fatty (OLETF) rats. Contractile response to electrical stimulation was decreased in the experimental groups compared with the control groups at 40 and 60 weeks, and the O-60 group had a lower contractile response than the O-40 group. The data are expressed as mean± SEM. L-40 group: LETO rats 40 weeks after birth (n=7), O-40 group: OLETF rats 40 weeks after birth (n=7), L-60 group: LETO rats 60 weeks after birth (n=7), O-60 group: OLETF rats 60 weeks after birth (n=7). a: significantly different from the control groups (p<0.05), b: significantly different from the O-40 group (p<0.05). |
 | Fig. 4Contractile response of bladder muscle strip to bethanechol (250µM) in Long Evans Tokushima Otsuka (LETO) and Otsuka Long Evans Tokushima Fatty (OLETF) rats. The contractile response to bethanecol (250µM) was decreased in the experimental groups compared with the control groups at 40 and 60 weeks, and the O-60 group had a lower contractile response than the O-40 group. The data are expressed as mean±SEM. L-40 group: LETO rats 40 weeks after birth (n=7), O-40 group: OLETF rats 40 weeks after birth (n=7), L-60 group: LETO rats 60 weeks after birth (n=7), O-60 group: OLETF rats 60 weeks after birth (n=7). a: significantly different from the control groups (p<0.05), b: significantly different from the O-40 group (p<0.05). |
 | Fig. 5Contractile response of the bladder muscle strip to ATP (10 mM) in Long Evans Tokushima Otsuka (LETO) and Otsuka Long Evans Tokushima Fatty (OLETF) rats. The contractile response to ATP (10 mM) was decreased in the experimental groups compared with the control groups at 40 and 60 weeks, and the O-60 group had a lower contractile response than the L-40 group. The data are expressed as mean±SEM. L-40 group: LETO rats 40 weeks after birth (n=7), O-40 group: OLETF rats 40 weeks after birth (n=7), L-60 group: LETO rats 60 weeks after birth (n=7), O-60 group: OLETF rats 60 weeks after birth (n=7). a: significantly different from the control groups (p<0.05), b: significantly different from the O-40 group (p<0.05). |
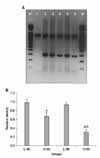 | Fig. 6(A) Reverse transcript-polymerase chain reaction (RT-PCR) measurement of nerve growth factor (NGF) mRNA expression. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used for the internal control. M: 100 bp marker; lane 1: GAPDH (983 bp); lane 2: GAPDH+NGF (294 bp); lane 3: L-40 group; lane 4: O-40 group; lane 5: L-60 group; lane 6: O-60 group. (B) Results of densitometric scanning of NGF mRNA expression. The mRNA expression of NGF was decreased in the O-40 and O-60 groups compared with the control groups. The mRNA expression of NGF was lower in the O-60 group than in the O-40 group. The data are expressed as mean±SEM. L-40 group: Long Evans Tokushima Otsuka (LETO) rats 40 weeks after birth (n=7), O-40 group: Otsuka Long Evans Tokushima Fatty (OLETF) rats 40 weeks after birth (n=7), L-60 group: LETO rats 60 weeks after birth (n=7), O-60 group: OLETF rats 60 weeks after birth (n=7). a: significantly different from the control groups (p<0.05), b: significantly different from the O-40 group (p<0.05). |
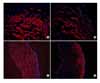 | Fig. 7Nerve growth factor (NGF) immunohistochemical staining of the bladder. NGF expression was decreased in the O-40 and O-60 groups compared with the control groups. NGF expression of the O-40 group was lower than in the O-60 group. (A) L-40 group (scale bar=25µm), (B) O-40 group (scale bar=25µm), (C) L-60 group (scale bar=25µm), (D) O-60 group (scale bar=25µm). L-40 group: Long Evans Tokushima Otsuka (LETO) rats 40 weeks after birth (n=7), O-40 group: Otsuka Long Evans Tokushima Fatty (OLETF) rats 40 weeks after birth (n=7), L-60 group: LETO rats 60 weeks after birth (n=7), O-60 group: OLETF rats 60 weeks after birth (n=7). |
References
1. Frimodt-Moller C. Diabetic cystopathy: epidemiology and related disorders. Ann Intern Med. 1980. 92:318–321.
2. Yoshimura N, Chancellor MB, Andersson KE, Christ GJ. Recent advances in understanding the biology of diabetes-associated bladder complications and novel therapy. BJU Int. 2005. 95:733–738.
3. Koo HP, Santarosa RP, Buttyan R, Shabsigh R, Olsson CA, Kaplan SA. Early molecular changes associated with streptozotocin-induced diabetic bladder hypertrophy in the rat. Urol Res. 1993. 21:375–381.
4. Steinbacher BC Jr, Nadelhaft I. Increased levels of nerve growth factor in the urinary bladder and hypertrophy of dorsal root ganglion neurons in the diabetic rat. Brain Res. 1998. 782:255–260.
5. Kim NS, Moon OR, Kang JH, Lee SY, Jeong BG, Lee SJ, et al. Increasing prevalence of obesity related disease for Koreans associated with overweight and obesity. Korean J Prev Med. 2001. 34:309–315.
6. Kawano K, Hirashima T, Mori S, Natori T. OLETF (Otsuka Long-Evans Tokushima Fatty) rat: a new NIDDM rat strain. Diabetes Res Clin Pract. 1994. 24:Suppl. S317–S320.
7. Kim JC, Seo SI, Park YH, Hwang TG. Changes of detrusor contractility and growth factors in streptozotosin-induced NIDDM rat. Korean J Urol. 2000. 41:615–621.
8. Andersson PO, Malmgren A, Uvelius B. Cystometrical and in vivo evaluation of urinary bladder function in rats with streptozotocin-induced diabetes. J Urol. 1988. 139:1359–1362.
9. Longhurst PA, Belis JA. Abnormalities of rat bladder contractility in streptozotocin-induced diabetes mellitus. J Pharmacol Exp Ther. 1986. 238:773–777.
10. Eika B, Levin RM, Longhurst PA. Collagen and bladder function in streptozotocin-diabetic rats: effects of insulin and aminoguanidine. J Urol. 1992. 148:167–172.
11. Lincoln J, Crockett M, Haven AJ, Burnstock G. Rat bladder in the early stages of streptozotocin-induced diabetes: adrenergic and cholinergic innervation. Diabetologia. 1984. 26:81–87.
12. Steers WD, Mackway AM, Ciambotti J, de Groat WC. Effects of streptozotocin-induced diabetes on bladder function in the rat. J Urol. 1990. 143:1032–1036.
13. Longhurst PA, Kauer J, Levin RM. The ability of insulin treatment to reverse or prevent the changes in urinary bladder function caused by streptozotocin-induced diabetes mellitus. Gen Pharmacol. 1991. 22:305–311.
14. Chung YG, Yoo HG, Kwon YH, Park CS, Lim WS, Ryu JK, et al. The significance of periurethral fibrosis and the change of nitric oxide synthase containing nerves in the urethra of diabetic rats. Korean J Urol. 2007. 48:1050–1057.
15. Kang DI, Kim SH, Lee SD, Kwak HS, Choi SH, Kim DR, et al. Effects of Alpha-lipoic acid on nitric oxide synthase expression and ultrastructural changes in the bladder of rats with streptozotocin-induced diabetes. Korean J Urol. 2007. 48:212–218.
16. Starer P, Libow L. Cystometric evaluation of bladder dysfunction in elderly diabetic patients. Arch Intern Med. 1990. 150:810–813.
17. Kaplan SA, Te AE, Blaivas JG. Urodynamic findings in patients with diabetic cystopathy. J Urol. 1995. 153:342–344.
18. Kanda M, Eto K, Tanabe N, Sugiyama A, Hashimoto K, Ueno A. Effects of ONO-2235, an aldose reductase inhibitor, on muscarinic receptors and contractile response of the urinary bladder in rats with streptozotocin-induced diabetes. Jpn J Pharmacol. 1997. 73:221–228.
19. Ordonez G, Fernandez A, Perez A, Sotelo J. Low contents of nerve growth factor in serum and submaxillary gland of diabetic mice. A possible etiological element of diabetic neuropathy. J Neurol Sci. 1994. 121:163–166.
20. Fernyhough P, Diemel LT, Brewster WJ, Tomlinson DR. Deficits in sciatic nerve neuropeptide content coincide with a reduction in target tissue nerve growth factor messenger RNA in streptozotocin-diabetic rats: effects of insulin treatment. Neuroscience. 1994. 62:337–344.
21. Fernyhough P, Diemel LT, Brewster WJ, Tomlinson DR. Altered neurotrophin mRNA levels in peripheral nerve and skeletal muscle of experimentally diabetic rats. J Neurochem. 1995. 64:1231–1237.
22. Jakobsen J, Brimijoin S, Skau K, Sidenius P, Wells D. Retrograde axonal transport of transmitter enzymes, fucose-labeled protein, and nerve growth factor in streptozotocin-diabetic rats. Diabetes. 1981. 30:797–803.
23. Schmidt RE, Modert CW, Yip HK, Johnson EM Jr. Retrograde axonal transport of intravenously administered 125I-nerve growth factor in rats with streptozotocin-induced diabates. Diabetes. 1993. 32:654–663.
24. Hellweg R, Raivich G, Hartung HD, Hock C, Keutzberg GW. Axonal transport of endogenous nerve growth factor (NGF) and NGF receptor in experimental diabetic neuropathy. Exp Neurol. 1994. 130:24–30.
25. Hellweg R, Hartung HD. Endogenous levels of nerve growth factor (NGF) are altered in experimental diabetes mellitus: a possible role for NGF in the pathogenesis of diabetic neuropathy. J Neurosci Res. 1990. 26:258–267.
26. Sasaki K, Yoshimura N, Chancellor MB. Implications of diabetes mellitus in urology. Urol Clin North Am. 2003. 30:1–12.
27. Pittenger G, Vinik A. Nerve growth factor and diabetic neuropathy. Exp Diabesity Res. 2003. 4:271–285.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download