Abstract
Purpose
We studied the feasibility and results of a tissue-engineered ileal conduit using a poly (ε-caprolactone) (PCL) nano-sheet seeded with muscle-derived stem cells to replace a conventional ileal conduit in rats.
Materials and Methods
Muscle-derived stem cells were isolated from the gastrocnemius muscle of female Sprague-Dawley rats (200-250 g, n=6) by use of a preplate technique and were cultured on a PCL nano-sheet. The PCL nano-sheet was implanted into the omentum of rats and was then made into a conical shaped conduit. Rats were sacrificed 4 and 8 weeks after implantation, and morphologic changes were assessed by H&E and immunofluorescence staining, including DAPI staining and staining for myogenin and myosin heavy chain (MyHC).
Results
All rats survived until the end of the experiment. A minimal inflammatory reaction was observed around the PCL nano-sheet in the 4 week specimens but was found to be reduced in the 8 week specimen. Muscle bundles were identified at week 4 as well as week 8 after implantation on H&E staining. Around the PCL sheet, immunostaining for both myogenin and MyHC were positive, indicating skeletal muscle differentiation and ingrowth into the PCL sheet.
Conclusions
A PCL nano-sheet seeded with muscle-derived stem cells showed successful skeletal muscle differentiation at 4 and 8 weeks after implantation. This preliminary result supports the feasibility of a tissue-engineered ileal conduit using a PCL nano-sheet (seeded with muscle-derived stem cells) in place of conventional ileal conduits.
REFERENCES
1.Kim WJ., Chung JI., Hong JH., Kim CS., Jung SI., Yoon DK. Epidemiological study for urologic cancer in Korea (1998-2002). Korean J Urol. 2004. 45:1081–8.
2.Herr HW., Shipley WU., Bajorin DF. Cancer of the bladder. De Vita VT, Hellman S, Rosenberg SA, editors. editors.Cancer: principles and practice of oncology. 6th ed.Philadelphia: Lippincott Williams & Wilkins;2001. 1396-7.
3.Park KK., Song JM. Bladder preservation management for muscle invasive bladder cancer. Korean J Urol. 2004. 45:19–23.
4.Chang SS., Cookson MS., Baumgartner RG., Wells N., Smith JA Jr. Analysis of early complications after radical cystectomy: results of a collaborative care pathway. J Urol. 2002. 167:2012–6.

5.Park HK., Lee SW., Yeo WG., Kwak C., Byeon SS., Kim HH, et al. Analysis of risk factors for ileus following radical cystectomy: Is the prolonged use of a nasogastric tube necessary? Korean J Urol. 2004. 45:1215–8.
6.Inman BA., Harel F., Tiguert R., Lacombe L., Fradet Y. Routine nasogastric tubes are not required following cystectomy with urinary diversion: a comparative analysis of 430 patients. J Urol. 2003. 170:1888–91.

7.Filmer RB., Spencer JR. Malignancies in bladder augmentations and intestinal conduits. J Urol. 1990. 143:671–8.

8.Khoury JM., Timmons SL., Corbel L., Webster GD. Complications of enterocystoplasty. Urology. 1992. 40:9–14.

10.Kambic H., Kay R., Chen JF., Matsushita M., Harasaki H., Zilber S. Biodegradable pericardial implants for bladder augmentation: a 2.5-year study in dogs. J Urol. 1992. 148:539–43.

11.Kropp BP., Eppley BL., Prevel CD., Rippy MK., Harruff RC., Badylak SF, et al. Experimental assessment of small intestinal submucosa as a bladder wall substitute. Urology. 1995. 46:396–400.

12.Probst M., Piechota HJ., Dahiya R., Tanagho EA. Homologous bladder augmentation in dog with the bladder acellular matrix graft. BJU Int. 2000. 85:362–71.

13.Byun SS., Kim JH., Oh JE., Kim HH., Lee E., Lee C. Short-term morphological and growth factor changes in rat bladders augmented with a porcine small intestinal submucosa. Korean J Urol. 2003. 44:473–80.
14.Byun SS., Lee JY., Ghil SH., Lee SS., Lee JH., Yook SH, et al. Preliminary study of tissue engineered bladder regeneration with poly (ε-caprolactone) (PCL) sheet seeded with autologous muscle-derived stem cell. Korean J Urol. 2005. 46:1094–7.
15.Cho SH., Oh SH., Lee KH., Kim HY., Lee JH. Preparation of polycarprolactone nanofibrous sheets by electrospinning and their cell adhesion and growth behavior. Biomater Res. 2003. 7:241–7.
16.Park JS., Kim S., Han DK., Lee JY., Ghil SH. Isolation of neural precursor cells from skeletal muscle tissues and their differentiation into neuron-like cells. Exp Mol Med. 2007. 39:483–90.

17.Lee JY., Paik SY., Yuk SH., Lee JH., Ghil SH., Lee SS. The isolation and characterization of muscle derived stem cells from gastrocnemius muscle of rats using the modified preplate method. Korean J Urol. 2004. 45:1279–84.
18.Danielsson C., Ruault S., Basset-Dardare A., Frey P. Modified collagen fleece, a scaffold for transplantation of human bladder smooth muscle cells. Biomaterials. 2006. 27:1054–60.

19.Hara I., Miyake H., Hara S., Gotoh A., Nakamura I., Okada S, et al. Health-related quality of life after radical cystectomy for bladder cancer: a comparison of ileal conduit and orthotopic bladder replacement. BJU Int. 2002. 89:10–3.

20.Hart S., Skinner EC., Meyerowitz BE., Boyd S., Lieskovsky G., Skinner DG. Quality of life after radical cystectomy for bladder cancer in patients with an ileal conduit, or cutaneous or urethral Kock pouch. J Urol. 1999. 162:77–81.

21.Kitamura H., Miyao N., Yanase M., Masumori N., Matsukawa M., Takahashi A, et al. Quality of life in patients having an ileal conduit, continent reservoir or orthotopic neobladder after cystectomy for bladder carcinoma. Int J Urol. 1999. 6:393–9.

22.Dahi DM., McDougal WS. Use of intestinal segments in urinary diversion. Kavoussi LR, Novick AC, Partin AW, Peters CA, Wein AJ, editors. editors.Campbell-Walsh urology. 9th ed.Philadelphia: Saunders;2007. 2534-78.
23.Jeong SI., Kim BS., Lee YM., Ihn KJ., Kim SH., Kim YH. Morphology of elastic poly (L-lactide-co-epsilon-caprolactone) copolymers and in vitro and in vivo degradation behavior of their scaffolds. Biomacromolecules. 2004. 5:1303–9.
25.Oberpenning F., Meng J., Yoo JJ., Atala A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat Biotechnol. 1999. 17:149–55.

Fig. 1.
Diagram of the poly (ε-caprolac-tone) (PCL) nano-sheet conduit surgical procedure. The PCL nano-sheet was attached to the omentum and made into a conical shape. The conical base of the conduit was opened for outflow of urine. Both ureters were dissected and mobilized level of renal hilum and ureteroneocystostomy were done. The conical base of the PCL nano-sheet conduit was secured to the rat’s abdominal skin.
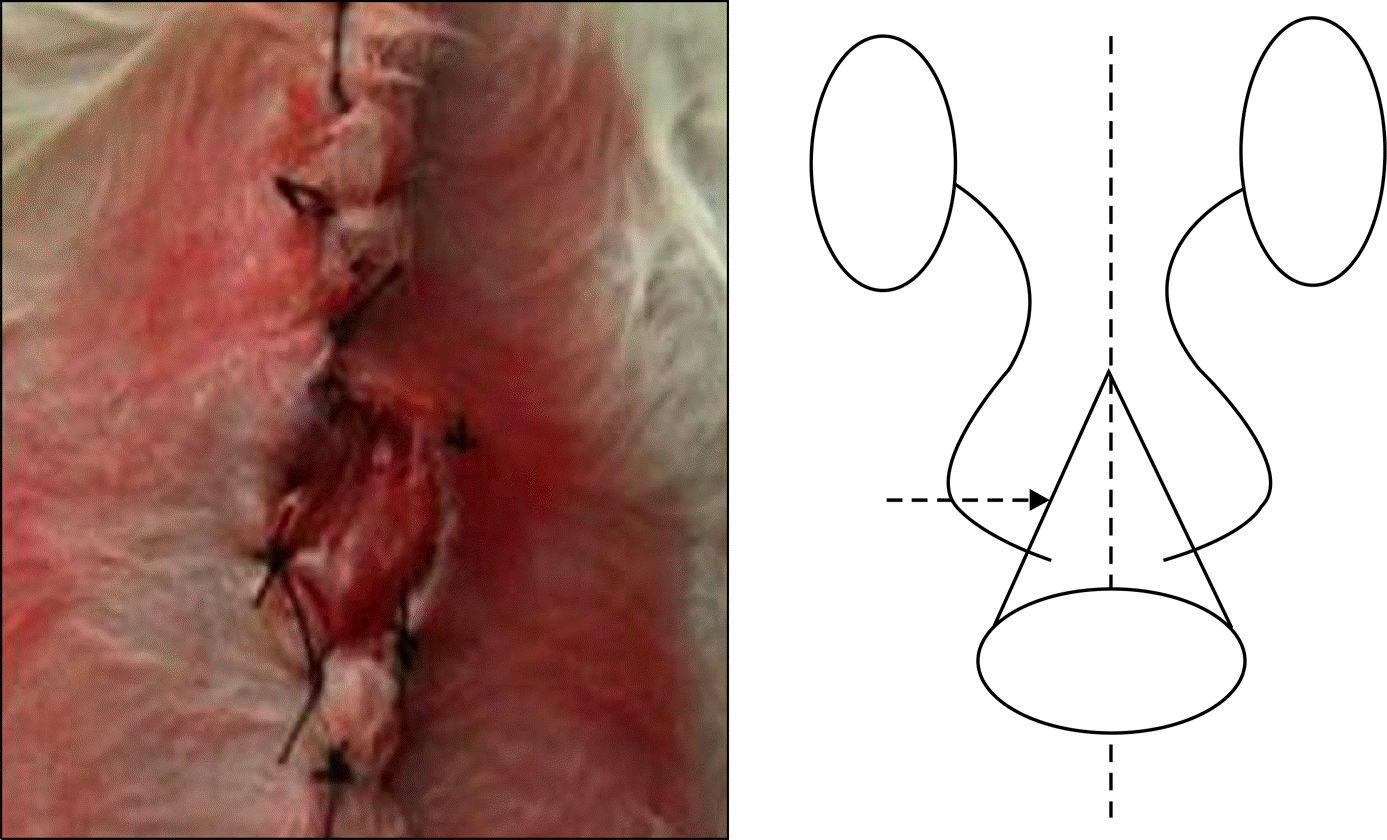
Fig. 2.
Histologic aspects of the muscle fibers on the poly (ε-caprolactone) (PCL) nano-sheet in the mesentery at 4 weeks after implantation. (A) Muscular bundles on the PCL nano-sheet can be seen (H&E, x100). (B) Neutrophils and lymphocytes can be seen around the PCL nano-sheet (H&E, x400). (C) PKH26 immunostaining shows differentiated skeletal muscle cells (red color) on the PCL nano-sheet and omental tissue after cryosection (x100). (D) DAPI immunostaining shows positive cells surrounding the sheet after cryosection (x100). (E) Immunostaining of myogenin and myosin heavy chain shows skeletal muscle bundle fibers (green color, x100).
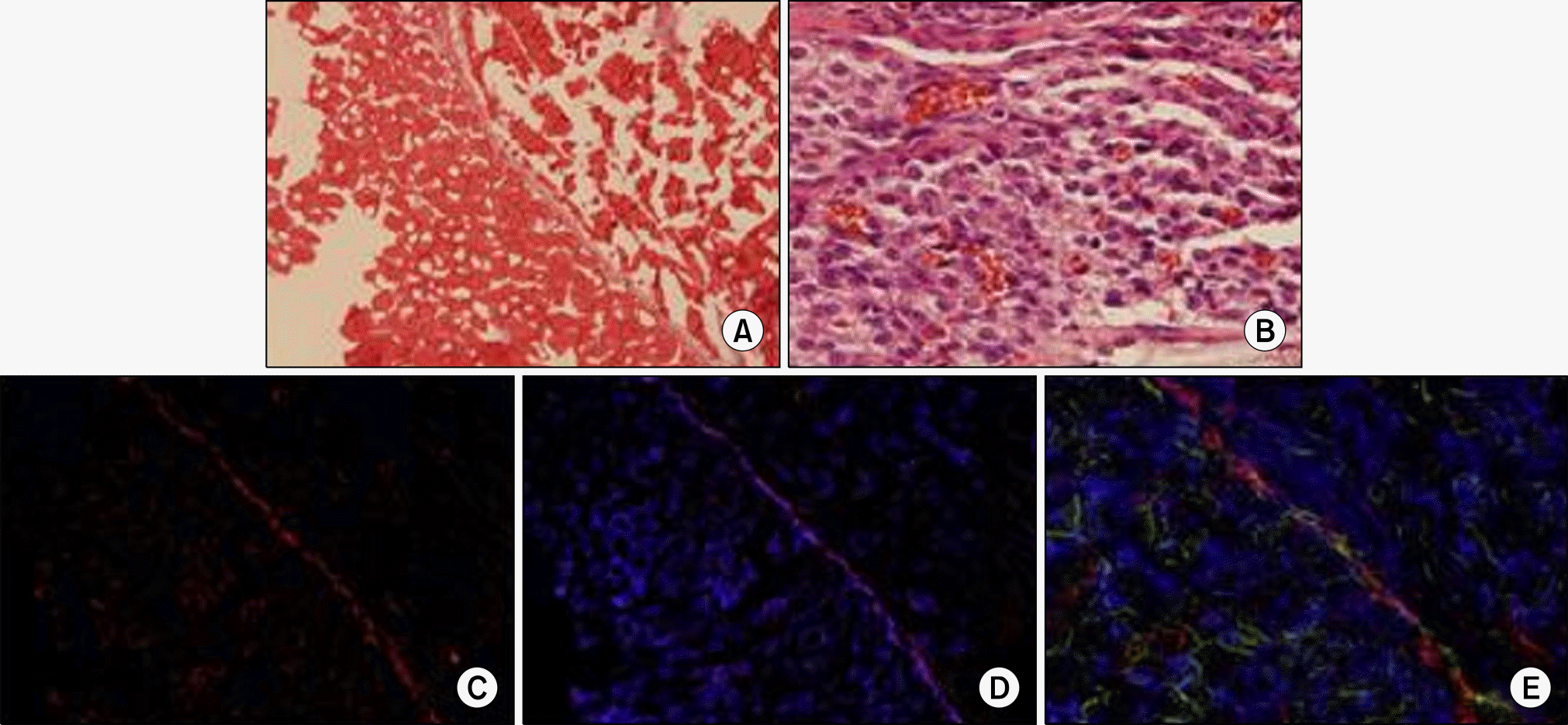
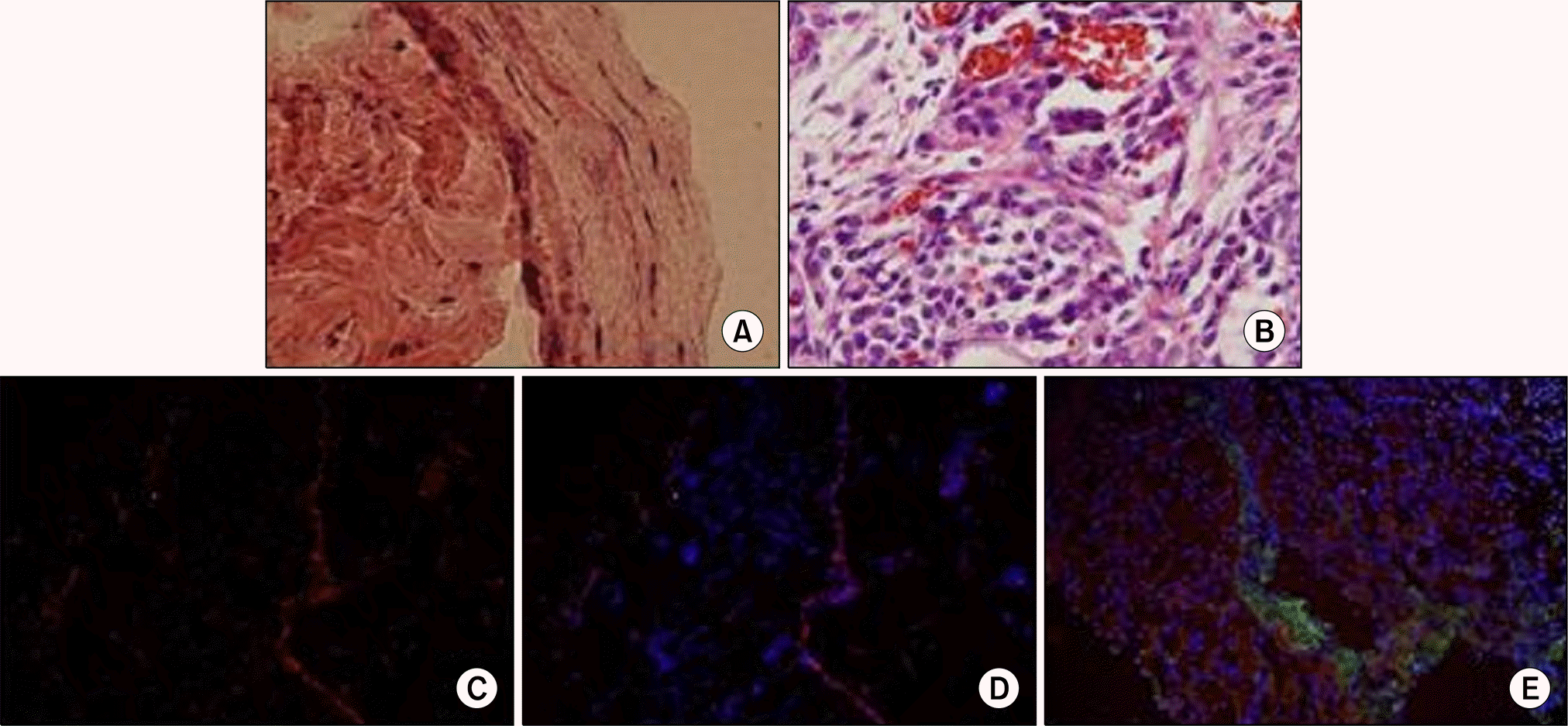
Fig. 3.
Histologic aspects of the muscle fibers on the poly (ε -caprolactone) (PCL) nano-sheet in the mesentery at 8 weeks after implantation. (A) Increased muscular bundles on the PCL nano-sheet can be seen (H&E, x100). (B) Decreased inflammatory cells around the PCL nano-sheet (H&E, x400). (C) PKH26 immunostaining shows the spread of differentiated skeletal muscle cells (red color) on the PCL nano-sheet and omental tissue after cryosection (x100). (D) DAPI immunostaining shows increased positive cells surrounding the sheet after cryosection (x100). (E) Immunostaining of myogenin and myosin heavy chain shows increased various types of cells and thick skeletal muscle bundle fibers (green color, x100).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download