Abstract
Purpose
Diabetes profoundly and negatively impacts all domains of female sexual function, however, the underlying pathophysiological mechanisms remain unknown. To date, limited studies have been published on the effects of type 1 & type 2 diabetes on female genital sexual arousal and how this may impact overall sexual function. The aim of this review is to discuss the effects of diabetes on female sexual function and insights from laboratory studies on the underlying pathophysiology.
Materials and Methods
Using PubMed, we reviewed and evaluated the literature published between 1970 and 2009 and data from our laboratories and others investigating the effects of type 1 and type 2 diabetes on genital sexual arousal responses.
Results
Women with diabetes experience diminished genital arousal, reduced vaginal lubrication, vaginal atrophy, dyspareunia, and increased vaginal infections. Also, a number of studies using type 1 and type 2 diabetic animal models have reported reduced plasma estradiol levels and marked physiological, biochemical and histological changes in genital tissues. In animal studies, diabetes alters genital tissue structure and attenuates expression of the estrogen, progesterone and androgen receptors and alters vaginal and clitoral hemodynamics. Importantly, treatment of diabetic animals with estradiol in the face of persistent hyperglycemia can restore vaginal structure and sex steroid receptor expression.
Conclusions
Type 1 & type 2 diabetic complications produce significant structural and functional disruptions in genital organs and attenuate genital hemodynamics. In the type 2 animal model, estradiol treatment ameliorates diabetic-induced pathophysiological alterations in genital tissues, such as the vagina. This suggests that estrogen supplementation may be beneficial in restoring diabetes-induced genital pathology.
The effects of diabetes on women's sexual health have received limited attention.1-3 In 1971, Kolodny4 first suggested a possible link between women's sexual dysfunction and diabetes. Although the effects of diabetes are well known to contribute to erectile dysfunction,5 relatively limited clinical epidemiological studies have addressed the etiology of sexual dysfunction in women. Some reports, however, point to similar deleterious effects of diabetes on female sexual function. Diabetes causes vasculopathies, neuropathies, and increases the likelihood of vaginal infections, all of which are integral to sexual health.6-8 Accumulating evidence strongly suggests that diabetes has a profound impact on female sexual function and well being.9-11
Jovanovic12 pointed to the considerable disparity that existed in efforts dedicated to studies of diabetes on male versus female sexual dysfunction. He noted that between 1997 to 2002, approximately 1,983 articles were published on diabetes and male sexual dysfunction, compared to only 13 articles published on diabetes and female sexual dysfunction. This illustrates the urgent need for more research in this field.
The estimated prevalence of sexual dysfunction in diabetic women varies between 14-51%.1,2 Approximately 27% of type 1 diabetic women experienced sexual dysfunctions compared to 15% of control subjects, with a significant number of women complaining of decreased lubrication.1,2 In addition, the prevalence of sexual dysfunctions was reported to be consistently higher in type 2 diabetic women with a higher incidence of decreased libido (77%), compared to control subjects (20%).13 Symptoms most commonly reported by diabetic women included loss of libido, diminished clitoral sensitivity, decreased vaginal lubrication, and increased vaginal discomfort.2,13-17 More recently, Doruk et al,18 reported an increased incidence of sexual dysfunctions including diminished arousal in type 2 diabetic patients and decreased arousal, lubrication and orgasm in type 1 diabetic women. In a recent study by Abu Ali et al,19 diabetic women aged 50 years or older were reported to have a significantly higher prevalence of decreased sexual desire than their nondiabetic age-matched counterparts (79.5% vs. 59%). Depression was also reported to be a predictor of decreased desire in diabetic women.20 Fatemi and Taghavi21 reported that sexual drive, arousal, vaginal lubrication, orgasm and overall satisfaction in Iranian women were all significantly lower in diabetic women, as assessed by sexual function questionnaires. Similar findings were reported in Nigerian22 and Peruvian women.23
The prevalence rate for orgasmic disorders in diabetic women has been estimated to be between 11-35%.4,15,16 The type of diabetes may also have an impact on the incidence of orgasmic disorder. Schreiner-Engel16 showed that type 1 diabetic women did not report reduced frequency of orgasm, whereas type 2 diabetic women reported significantly less frequent orgasms when compared to control subjects. Similarly, Enzlin et al2 found that type 1 diabetic women, both with and without diabetic complications, did not report problems with orgasm. In contrast, Doruk et al,18 reported no significant differences with regard to orgasm in type 2 diabetic women, whereas orgasm was significantly reduced in type 1 diabetic women when compared to healthy, control subjects. These limited and conflicting studies suggest that sexual orgasmic disorder is frequent in diabetic women, but that the type (and potentially, duration) of diabetes may have a differential impact on sexual function.
Dyspareunia is the most common form of sexual pain disorder in women and its estimated prevalence rate varies between 3-16% in the diabetic population.4,16,20,24,25 Diabetic women also tend to experience an increased prevalence of vaginal infections and decreased vaginal lubrication which may contribute to their symptoms of sexual pain disorder.26
Although clinical and epidemiological studies suggest that diabetes contributes to female sexual dysfunctions, limited pre-clinical studies are available to document changes in the various types of sexual dysfunction with diabetes. This is attributed in part to lack of appropriate animal models of diabetes to investigate female sexual function. In addition, few basic science studies have detailed the physiological mechanisms of normal female sexual arousal, and current animal models have inherent limitations in the investigation of sexual desire and orgasmic function. Thus, animal studies have focused on the effects of diabetes on the structural and functional changes in the genitals that might affect the female genital sexual arousal response. Yet, even these types of studies are few in number and limited in scope.
The vagina is an organ involved in the peripheral genital arousal response. Fluctuations in estradiol levels are known to induce structural and functional changes in the vagina and there is emerging evidence that diabetes induces changes in steroidogenesis, specifically that of estradiol, which may negatively affect vaginal function.27-33 The aim of this review is to summarize data derived from clinical and laboratory studies pertaining to the effects of types 1 & 2 diabetes on female sexual function in general, and in particular, on the effects of diabetes on female genital sexual arousal, as manifested by alterations in the structure and function of genital tissues. We hope to shed some light on the potential role of diabetes in disrupting endocrine modulation of genital tissues, and the potential amelioration of this pathology by hormone treatment.
A number of studies have reported histologic and/or physiologic changes in genital tissues of type 1 diabetic animals.34-36 Interestingly, diabetic animals had reduced plasma estradiol levels and increased testosterone levels.36-39 Similarly, studies suggest that type 2 diabetes reduces circulating estradiol levels.38-41 Further, data from our laboratory have demonstrated that type 2 diabetic female mice have reduced estradiol levels and increased testosterone levels with testosterone to estradiol ratio higher in the diabetic animals than in control or estrogen treated diabetic animals (Fig. 1). Reduced estradiol levels were consistent with marked uterine and vaginal atrophy in untreated diabetic animals.
Ovarian aromatase activity has been shown to be insulindependent 42-44 and reduced aromatase expression and/or interference with its functional activity may result in decreased estradiol levels in diabetes. The aromatase enzyme is essential in the conversion of 4-androstenedione and testosterone to estrone and estradiol in the adrenal glands, ovaries, placenta, testicles, adipose (fat) tissue, and brain. In studies of women with type 1 diabetes, it has been shown that the ovaries have reduced ability to convert androgen to estrogen, probably due to a reduction of ovarian aromatase activity.43 Additionally, it has been reported that treatment with metformin, an anti-diabetic drug which acts by suppressing hepatic gluconeogenesis,45 is associated with a reduction in aromatase activity in response to FSH stimulation42,44 have further shown that high levels of glucose lead to decreased estradiol production via decreased aromatase activity.42 Thus, the observed increase in testosterone and decrease in estradiol levels in several studies may be a result of decreased ovarian aromatase activity in diabetes.36,38,39
Studies in diabetic animals demonstrated marked changes in vaginal and clitoral histo-architecture34,36,46 and reductions of key biochemical markers known to be regulated by estrogen, such as estrogen receptor alpha (ERα), androgen receptor (AR), and arginase.34,36 Diabetes resulted in reduced vaginal muscularis layer and less uniform collagen deposition, (Fig. 2). Furthermore, Park et al35 demonstrated a significant increase in clitoral cavernosal fibrosis in a type 1 diabetic animal model. Diabetes has also been implicated in vaginal fibrosis, as evidenced by increases of profibrotic factors including TGF-β 1 and plasminogen activator inhibitor (PAI-1) expression.34,46 Diabetic-induced changes increased collagen deposition and reduced thickness of the vaginal epithelium in type 1 diabetic animals.34,46 These histological findings may, in part, explain reduced vaginal compliance, increased trauma and diminished arousal in diabetic women. The increased deposition of fibrotic elements in vaginal tissues as a result of the diabetic insult may further contribute to this pathology.2,13,16,18
Recent studies from our laboratory have shown that type 2 diabetic animals also exhibited dramatic alterations in vaginal histo-architecture (Fig. 3).47 The vaginal wall appeared significantly thinner and the epithelium was markedly atrophic and resembled that of estrogen-deficient animals. Furthermore, the epithelial cells were smaller in size than those from the epithelium of control animals (unpublished observations). Thinning of the epithelium and loss of its outer zones, which were prominent histological features of the type 2 diabetic and muscular atrophy evidenced in the vagina in diabetic animals, suggest possible disruptions in cell proliferation and/or growth. Diabetes has been shown to disrupt cell growth and proliferation and induce apoptosis in the pancreas,38 heart,48 kidney,49 retina,50 and spinal cord.51
The vaginal epithelium has important functions in vaginal lubrication (e.g., permeability and the production of mucin glycoproteins), thought to be regulated by estrogens.52 The existence of aquaporins (water channel proteins) have been demonstrated in rat vaginal epithelium,53 but the effects of estrogen or diabetes on their expression, distribution and function have not been directly determined. Diabetes-induced changes in epithelial structure are expected to alter functional responses in genital tissues, including increased trauma and painful intercourse, decreased vaginal lubrication, and increased infections. Normal stratification of the epithelium protects the vaginal wall from abrasions and trauma during coitus. Thus, in the diabetic state, the loss or decrease of the physical barrier between the epithelium and underlying lamina propria where the sensory nerve fibers lie, may account for painful vaginal sensations experienced in diabetic patients.13,16 In addition, the superficial stratified layers of the epithelium impose a physical barrier to bacteria and fungi that richly populate the vaginal lumen. Glycogen within the superficial epithelial cells is broken down to produce lactic acid in order to maintain an acidic environment that suppresses bacterial and fungal infections. Human studies have shown that diabetic patients are at a higher risk for developing vaginal candidiasis.7
Alterations in the vaginal connective tissue elements and muscularis layer were also apparent in type 2 diabetic animals. Changes in the architecture of vaginal connective tissues included deposition of dense, compact connective tissue comprised of thicker, coarser and less uniform collagen fiber bundles coupled with a marked reduction and simplification of the elastic fiber network, notably a drastic truncation of the individual elastic fibers (unpublished observations). Similar diabetes-induced changes in elastic fiber networks are well documented in tissue of the corpora cavernosa of male patients where elastic fibers appear shortened or are absent in the tunica albuginea, ultimately contributing to erectile dysfunction.54 Structural and functional properties of connective tissues are well known to be affected in the diabetic state. Increased glomerulosclerosis and tubulointerstitial fibrosis in the diabetic kidney have been shown to lead to decreased renal function in rat and mouse models of diabetic disease.40,55
The smooth muscle of the vaginal wall plays an important role in the female sexual arousal response. In untreated diabetic animals, the vaginal muscularis undergoes marked morphological changes and becomes atrophic and disorganized, and its volume reduced to roughly half that of control animals (Cushman et al, unpublished observations). These findings were concordant with those reported by Kim et al36 and Ferrini et al,46 who showed similar decreases of vaginal smooth muscle in type 1 diabetic rats. Additionally, clitoral cavernous smooth muscle was reduced in alloxan-induced type 1 diabetic rabbits, compared to controls.35 Furthermore, studies in ovariectomized animals have yielded similar observations where surgically-induced estrogen-deficiency resulted in atrophy of the vaginal epithelium and muscularis layers.29,30,56 Such marked degeneration of the muscularis, combined with changes in connective tissue content, composition and structure, are likely to alter the tonicity of the vaginal wall and modify vaginal compliance. The health of the smooth muscle layer is undoubtedly dependent upon the integrity of its nerve supply, the resident sex steroid hormone receptors and various other factors. While each of these modulatory influences require further characterization, changes in vaginal tissue compliance, contractile function and perfusion, secondary to the diabetic state may contribute to genital pain associated with sexual activity in diabetic women.
Estrogen receptor alpha (ERα) protein expression is significantly reduced in vaginal tissues from type 1 diabetic animals.36 This observation was further corroborated by reduction in ERα immunostaining and distribution throughout the vaginal wall from type 1 diabetic animals (unpublished observations). Similarly, expression of progesterone receptor (PR) (unpublished observations) and androgen receptor (AR) was significantly reduced in vaginal extracts from type 1 diabetic animals.36 Furthermore, while the distribution of PR in vaginal tissues from type 1 diabetic animals was similar to that of controls, the staining intensity and proportion of PR immunoreactive cells in the muscularis layer of vaginal tissues from diabetic animals was significantly reduced compared to controls (unpublished observations). Because the vagina is a target tissue for sex steroid hormones, it is likely that diabetes-induced changes in sex steroid receptor expression and function impedes normal physiological genital function.
In the vagina of type 2 diabetic animals, overall expression of ERα, PR and AR was markedly reduced and significant changes in receptor localization and distribution was also noted.47 Furthermore, type 2 diabetes resulted in significantly increased immunolocalization of steroid hormone receptors in the atrophic epithelium.57 This observation is similar to those reported by Pessina et al29,30 who showed similar increased immunolocalization of steroid hormone receptors in the atrophic epithelium of ovariectomized animals. We postulate that this may represent a biological process of compensation to maintain the epithelium at a functioning basal state in the diabetic milieu and prevent any further tissue involution.
Sexual arousal in the female is mediated by a host of physiological mechanisms including vascular smooth muscle relaxation via nitrergic neurotransmission which leads to increased vaginal blood flow and lubrication.26,28,58 The nitrergic neuronal subsystem plays an important role, not only in vasodilation, but also in the relaxation of the non-vascular smooth muscle of genital tissues.27,28,59,60 Immunostaining for neural nitric oxide synthase (nNOS) revealed that the nitrergic system of nerve fibers was significantly attenuated in type 1 & type 2 diabetic animals (Fig. 4, 5). When changes in the thickness of the vaginal wall due to diabetes are taken into account, the total extent of nitrergic innervation was drastically reduced in type 1 diabetic animals (Fig. 2, unpublished data). A significant reduction of the nitrergic population of nerve fibers was noted in vaginal tissue of type 2 diabetic animals (Fig. 5).57 When changes in the thickness of the vaginal wall are taken into account, the total extent of nitrergic innervation was drastically reduced in these diabetic animals. These findings were in accordance of Giraldi et al58,61 who reported significant attenuation in the nitrergic neurotransmission in vaginal tissues from type 1 diabetic animals. These histological findings corroborate the physiological findings of other studies. Pelvic nerve stimulation of diabetic animals resulted in reduced vaginal blood flow.36
Similarly, Park et al35 also demonstrated significantly reduced clitoral intracavernosal blood flow using a diabetic animal model. In organ bath assays of vaginal tissues precontracted with noradrenaline, the relaxing effect of SNAP (S-nitroso-N-acetylpenicillamine), an NO donor, was significantly reduced in tissues from type 1 diabetic rats, as compared to control animals.61 Furthermore, these observations are in accordance with those of El-Sakka et al62 and Cellek et al63 who showed significant decreases in NOS-containing nerve fibers in the dorsal and intracavernosal nerves from penile tissues in diabetic animals.62,63 In clinical studies, Caruso et al64,65 have reported that women with type 1 diabetes have sexual arousal disorder and that treatment with PDE 5 inhibitors increased clitoral blood flow in diabetic women. The significant atrophy of vaginal and clitoral tissues in untreated diabetic animals may be a result of altered estrogen action, either by reducing circulating estradiol levels and/or disrupting the expression and signaling of ERα. Thus, alterations in the nitrergic system of nerve fibers in the vagina may underlie reduced blood flow, the loss of vaginal sensation and/or dyspareunia, and decreased lubrication, all of which have been reported in diabetics in both human and animal studies.2,13,36
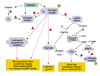
Considerable evidence is emerging from animal models and human studies suggesting that estrogens are a protective factor in several pathological states including cardiovascular (CVD) and renal diseases. Estrogens are generally associated with an anti-atherogenic and anti-oxidant profile of health. Interestingly, in diabetes, this protective factor is lost and diabetic women are at similar risk for CVD as non-diabetic men.66 Furthermore, the premenopausal advantage in clearance of dietary lipids is lost in premenopausal diabetic women, a condition likely to promote an atherogenic lipoprotein profile.67 Endothelial dysfunction, a possible early manifestation of atherogenesis, was more prominent in postmenopausal diabetic patients when compared to normal postmenopausal subjects.68 The incidence and rate of progression of renal disease were also shown to be far greater in nondiabetic men than nondiabetic women, further suggesting a protective role for estrogens.69 Moreover, premenopausal diabetic women have a lower risk of developing end-stage renal disease compared with age-matched diabetic males, whereas this protection is lost after menopause.70 Thus, these clinical studies strongly suggest a potential link between estrogens and progression of diabetic induced pathophysiology of estrogen-dependent tissues. Based on clinical and pre-clinical evidence, we advance the hypothesis that diabetes disrupts estrogen biosynthesis, estrogen receptor expression and function (Fig. 6).
If, as hypothesized above, estrogen decline is indeed indicated in the progression of diabetic disease, then estrogen replacement may reverse or restore diabetes-induced pathology. Interestingly, estrogen therapy has proven to be protective in a number of human and animal studies. Treatment of diabetic animals with 17β-estradiol attenuated diabetic nephropathy by reducing albuminuria, improving creatinine clearance, reducing glomerulosclerosis, tubulointerstitial fibrosis, and reducing TGF-β 1 expression in the renal cortex.71 In a group of healthy, nonobese postmenopausal women, transdermal 17β-estradiol users experienced a significant reduction in the incidence of type 2 diabetes when compared to "nonhormone users".72 In 2005, Garris and Garris38 reported that diabetes-induced hypercytolipidemia in pancreatic islet cells was markedly reduced following estrogen treatment along with the restoration of β-cell cytoarchitecture and cellular insulin concentrations. Low-dose estradiol treatment of postmenopausal women with type 2 diabetes significantly lowered fasting glucose and total cholesterol when compared to placebo treated postmenopausal women with type 2 diabetes.73 Therefore, studies suggest a protective and possible anti-diabetogenic role for estrogen therapy in women.
No studies to date have investigated the effects of estradiol supplementation on diabetes-induced complications in genital tissues. Given the importance of estradiol in the health and proper functioning of genital tissues, investigating the role of estrogens in diabetes with regard to genital physiology provides an interesting point of convergence for the physiology of steroid hormones and the pathophysiology of diabetes. Estrogen treatment of type 2 diabetic animals ameliorated the structural changes induced by diabetes in the vagina57 The vaginal epithelium became hyperplastic, and the muscularis layer was completely restored. Furthermore, diabetes-induced changes in the histological appearance of the connective tissues were partially attenuated by estradiol supplementation. Therefore, estradiol promotes recovery of connective tissue from alterations induced by diabetes.
Estradiol supplementation also partially reversed the diabetes-induced alterations in the nitrergic innervation of the vaginal wall. Tissue from estrogen-supplemented diabetic animals showed a trend towards re-establishment of the nitrergic nerve fiber network in the vaginal wall, suggesting that this population of nerve fibers may be regulated by estrogen (unpublished observationd). This has also been shown to be the case in oophorectomied animals whereby nNOS expression declined while estrogen replacement significantly increased nNOS protein expression.74 In addition, uterine arteries treated with 17-beta estradiol exhibited an increase in vascular smooth muscle nNOS mRNA protein expression.75 Thus, the data in our study57 suggests that treatment of diabetic animals with estradiol enhanced the density of nNOS immunopositive fibers. These findings implicate a functional role of estradiol in maintaining nNOS expression and the relaxant properties of the vascular and nonvascular smooth muscle of the vaginal wall.
We further observed that estradiol supplementation restored steroid hormone immunolocalization in the vagina in a differential manner.47 This finding suggests disruption of estrogen signaling in diabetes. This comes from several lines of evidence that include the dramatic changes in vaginal tissue structure, as well as the coincident changes in the levels of PR and AR. As described previously, vaginal tissue from type 1 and/or type 2 diabetic mice exhibited marked reductions of epithelial thickness and muscularis area whereas these diabetes-induced changes were ameliorated by estradiol supplementation. These atrophic changes observed in intact diabetic mice paralleled the structural alterations observed in ovariectomized rats.29,30 In addition, PR and AR expression has been shown to be regulated by the activity of ERα. Indeed, regulation of the progesterone receptor has been shown to be tightly coupled to estrogen signaling. In ovariectomized animals, estradiol treatment has been shown to increase PR in vaginal epithelial cells via ERα signaling.76 Similar studies have also reported PR expression levels to decrease after ovariectomy and be restored with estradiol treatment.29,30,77,78 Thus, increased PR expression in type 2 diabetic animals by estradiol supplementation strongly suggests restoration of estrogen receptor signaling.
With respect to the AR, its actions have also been shown to be regulated by estradiol. Pelletier et al79 have shown that ovariectomy decreases AR mRNA levels in the mouse uterus and vagina, while estradiol treatment restores AR expression to levels observed in control animals, suggesting a positive estrogenic regulation of AR in these tissues.79,80 Furthermore, it has been demonstrated in ovariectomized animals that AR levels were reduced in the proximal and distal vagina, whereas expression was restored in estrogen-treated animals.81 The decreased levels of androgen receptor in the type 1 and type 2 diabetic animals suggest that estrogen action may be disrupted in the diabetic state. Therefore, estrogenic restoration of AR expression and distribution also suggests re-establishment of estrogen receptor signaling in diabetic animals.
Our findings are in agreement with previous studies that have correlated changes in steroid hormone receptors with the diabetic state. Ekka et al76 showed similar estrogen-induced progesterone receptor expression in uterine tissues in a type 1 diabetic rat animal model.76 Interestingly in our studies,47,57 estrogen supplementation (at supraphysiologic levels), in diabetic animals, down-regulated ERα expression beyond levels detected in untreated diabetic animals. However, this observation is also consistent with studies in female rats in which estradiol treatment of ovariectomized animals down-regulated ERα in vaginal tissues.29,30 Thus, the data from the present study suggests that types 1 and 2 diabetes decreased the expression of ERα as well as PR and AR in the vagina. The changes in steroid receptor expression, therefore, may underlie the observed diabetes-induced changes in the structural integrity of the vagina.
Evidence for a role of estradiol in energy metabolism and glucose homeostasis has been suggested by several human and animal studies.82-85 The exact cellular mechanisms involved in estradiol-mediated glucose metabolism remain to be elucidated. One possible mechanism is ERα regulation of the expression of glucose transporters. Several studies have suggested a possible link between estradiol and the expression of GLUT4.86,87 GLUT4 expression has been shown to be upregulated with estrogen treatment in primate cerebral cortex.88 ERα null mice showed reduced GLUT4 expression in skeletal muscle.86,87 Changes in GLUT4 expression and/or translocation to the plasma membrane may result in insulin resistance.86,87 Selective estrogen receptor modulators (SERMs) increase GLUT3 and GLUT4 mRNA expression levels in cerebral cortical neurons and may possess neuroprotective effects due to increased glucose transport.88 It is therefore possible that decreased estradiol levels and down regulation of ERα in the untreated diabetic (db/db) mice may contribute to a dysregulation of GLUT4 expression and consequently lead to altered glucose uptake at the level of insulin-sensitive tissues. This, however, remains to be established.
Studies in animal models of diabetic nephropathy suggest that estrogen regulates diabetes-induced fibrosis. Supplementation with 17β-estradiol attenuated diabetic renal disease by regulating extracellular matrix protein expression associated with glomerulosclerosis and tubulointerstitial fibrosis, TGF-β and its downstream regulatory proteins, and the activity of matrix metalloproteinases in renal tissues.40,89 17β-estradiol also suppresses renal mesangial expansion and fibronectin accumulation in diabetic db/db mice.55 Furthermore, ERα knockout mice exhibited proteinuria and glomerulonephritis suggesting a protective role for estrogen and its actions on renal tissues.90 Thus, these studies support a role for estradiol in ameliorating diabetes-induced fibrotic pathology.
Sex steroid hormone receptor signaling is important in female genital sexual arousal function. Estrogen biosynthesis and receptor signaling are altered in diabetes and therefore, alter vaginal physiological function. The current review of the literature and reporting of unpublished observations suggest that sex steroid hormone receptors are differentially regulated in diabetes with potential pathological consequences on vaginal physiology. Similarly, reduced expression of PR and AR may signify further alterations in vaginal physiology since AR is important in maintaining the adrenergic neural fiber network and nNOS expression.29,30,91 These findings further demonstrate that estradiol supplementation provides a protective effect by upregulating the expression of sex steroid receptor proteins.
Studies in humans and in animal models of type 1 and type 2 diabetes suggest that diabetic complications induce histological and physiological alterations in genital tissues which ultimately impair female genital sexual arousal response. As illustrated in Fig. 6, we postulate that diabetic complications may adversely affect vaginal health and function by altering sex steroid hormone production. In addition, alteration of ERα expression or binding properties could lead to decreased binding of estrogens and/or attenuated receptor signaling, resulting in decreased expression or activity of estrogen- dependent proteins within the vagina. In summary, based on the present literature review, type 1 and type 2 diabetes induce genital atrophy concomitant with structural and functional alterations in the various genital organs. Type 1 diabetes caused a significant reduction in blood flow to the clitoris and the vagina which is a critical component of the genital arousal response. The attenuated blood flow response is also accompanied by dramatic changes in tissue structure, innervation and key biochemical markers important to maintaining genital tissue integrity. These structural changes are ameliorated by estrogen treatment of diabetic animals. Further, estradiol supplementation provides a restorative effect by up-regulating the expression of sex steroid receptor proteins. These observations suggest that estrogen therapy may be beneficial in treating diabetes-induced genital structural and functional changes and restoring normal genital physiology and sexual arousal responses in diabetic women.
Figures and Tables
Fig. 1
Plasma estradiol and testosterone levels in control and diabetic mice. Plasma levels of estradiol and testosterone from control, diabetic (untreated) and diabetic animals treated with estradiol (E2) were determined by immunoassay at the Endocrinology Laboratory, Animal Health Diagnostic Center at the Cornell University College of Veterinary Medicine (Ithaca, USA), as described by Cushman et al.47,57 Note, that estradiol levels decreased in diabetic animals and testosterone levels increased in diabetic animals. This resulted in an imbalance in the testosterone to estradiol ratio (T/E) in diabetic animals. This ratio is normalized in diabetic animals treated with estradiol.

Fig. 2
Effects of type 1 diabetes on vaginal epithelial and muscularis morphology. Vaginal tissue sections from control and untreated diabetic were subjected to a Masson's trichrome staining protocol. The panels are representative sections from control (A) and untreated diabetic (B) animals. The 3 layers of the vaginal wall are labeled as epithelium (E), lamina propria (LP), muscularis (M). Scale bars represent 100 µm (magnification, ×200).
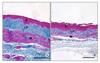
Fig. 3
Effects of type 2 diabetes and estrogen supplementation on vaginal epithelial and muscularis morphology. Vaginal tissue sections from control, untreated diabetic and diabetic animals treated with estradiol were subjected to a Masson's trichrome staining protocol. The panels are representative sections from control (A), untreated diabetic (B), and diabetic animals supplemented with estradiol (C). The 3 layers of the vaginal wall are labeled as epithelium (E), lamina propria (LP), muscularis (M). Scale bars represent 100 µm (magnification, ×200).

Fig. 4
Effects of type 1 diabetes on the density of neural nitric oxide synthase-immunopositive nerve fibers in vaginal tissue sections. Formalin-fixed and paraffin-embedded vaginal tissue sections from control (A) and untreated diabetic (B) animals were subjected to immunostaining procedures using anti-nNOS antibody and counterstained with Gill's hematoxylin (scale bar represents 100 µm, panels are at magnification, ×200). nNOS immunopositive nerve fibers are denoted by arrowheads. E: epithelium, LP: lamina propria, M: muscularis.
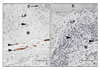
Fig. 5
Effects of type 2 diabetes and estradiol supplementation on the density of neural nitric oxide synthase (nNOS) immunopositive nerve fibers in vaginal tissue sections. Formalin-fixed and paraffin-embedded vaginal tissue sections from control (A), untreated diabetic (B), and estradiol treated diabetic (C) animals were subjected to immunostaining procedures using anti-nNOS antibody and counterstained with Gill's hematoxylin (scale bar represents 20 µm, panels are at magnification, ×400). nNOS immunopositive nerve fibers are denoted by arrowheads.

Fig. 6
Postulated effects of diabetes on estrogen production and signaling in the vagina. Diabetic complications may adversely affect vaginal health and function by altering sex steroid hormone production and/or steroid hormone receptor signaling (red triangles). Disruptions in ovarian function may lead to decreased production of estrogens. In addition, alteration of ERα expression or binding properties could lead to decreased binding of estrogens and/or attenuated receptor signaling, resulting in decreased expression or activity of estrogen-dependent proteins within the vagina. Direct interactions are represented by solid arrows, whereas indirect or multi-step processes are represented by dashed arrows. PKG: protein kinase G, PDE5: phosphodiesterase 5, cGMP: cyclic guanosine monophosphate, NO: nitric oxide, GTP: guanosine triphosphate, eNOS: endothelial nitric oxide synthase, nNOS: nitric oxide synthase, E2: estradiol (Adopted from Kim NN, J Sex Med 2009;6(Suppl 3):239-246).
References
1. Enzlin P, Mathieu C, Vanderschueren D, Demyttenaere K. Diabetes mellitus and female sexuality: a review of 25 years' research. Diabet Med. 1998. 15:809–815.
2. Enzlin P, Mathieu C, Van den Bruel A, Bosteels J, Vanderschueren D, Demyttenaere K. Sexual dysfunction in women with type 1 diabetes: a controlled study. Diabetes Care. 2002. 25:672–677.
3. Koch PB, Young EW. Diabetes and female sexuality: a review of the literature. Health Care Women Int. 1988. 9:251–262.
4. Kolodny RC. Sexual dysfunction in diabetic females. Diabetes. 1971. 20:557–559.
5. Fonseca V, Jawa A. Endothelial and erectile dysfunction, diabetes mellitus, and the metabolic syndrome: common pathways and treatments? Am J Cardiol. 2005. 96:13M–18M.
6. Bailes BK. Diabetes mellitus and its chronic complications. AORN J. 2002. 76:266–282.
7. de Leon EM, Jacober SJ, Sobel JD, Foxman B. Prevalence and risk factors for vaginal Candida colonization in women with type 1 and type 2 diabetes. BMC Infect Dis. 2002. 2:1.
8. DeUgarte CM, Berman L, Berman J. Female sexual dysfunction-from diagnosis to treatment. Sex Reprod Menopause. 2004. 2:139–145.
9. Grandjean C, Moran B. The impact of diabetes mellitus on female sexual well-being. Nurs Clin North Am. 2007. 42:581–592.
10. Rutherford D, Collier A. Sexual dysfunction in women with diabetes mellitus. Gynecol Endocrinol. 2005. 21:189–192.
11. Muniyappa R, Norton M, Dunn ME, Banerji MA. Diabetes and female sexual dysfunction: moving beyond "benign neglect". Curr Diab Rep. 2005. 5:230–236.
12. Jovanovic L. Finally, it is our turn! Diabetes Care. 2002. 25:787–788.
13. Erol B, Tefekli A, Ozbey I, Salman F, Dincag N, Kadioglu A, et al. Sexual dysfunction in type II diabetic females: a comparative study. J Sex Marital Ther. 2002. 28:Suppl 1. 55–62.
14. Wincze JP, Albert A, Bansal S. Sexual arousal in diabetic females: physiological and self-report measures. Arch Sex Behav. 1993. 22:587–601.
15. Jensen SB. Diabetic sexual dysfunction: a comparative study of 160 insulin treated diabetic men and women and an age-matched control group. Arch Sex Behav. 1981. 10:493–504.
16. Schreiner-Engel P, Schiavi RC, Vietorisz D, Smith H. The differential impact of diabetes type on female sexuality. J Psychosom Res. 1987. 31:23–33.
17. LeMone P. The physical effects of diabetes on sexuality in women. Diabetes Educ. 1996. 22:361–366.
18. Doruk H, Akbay E, Cayan S, Akbay E, Bozlu M, Acar D. Effect of diabetes mellitus on female sexual function and risk factors. Arch Androl. 2005. 51:1–6.
19. Abu Ali RM, Al Hajeri RM, Khader YS, Shegem NS, Ajlouni KM. Sexual dysfunction in Jordanian diabetic women. Diabetes Care. 2008. 31:1580–1581.
20. Enzlin P, Mathieu C, Van Den Bruel A, Vanderschueren D, Demyttenaere K. Prevalence and predictors of sexual dysfunction in patients with type 1 diabetes. Diabetes Care. 2003. 26:409–414.
21. Fatemi SS, Taghavi SM. Evaluation of sexual function in women with type 2 diabetes mellitus. Diab Vasc Dis Res. 2009. 6:38–39.
22. Olarinoye J, Olarinoye A. Determinants of sexual function among women with type 2 diabetes in a Nigerian population. J Sex Med. 2008. 5:878–886.
23. Mezones-Holguin E, Blümel JE, Huezo M, Vargas R, Castro J, Córdova W, et al. Impact of diabetes mellitus on the sexuality of Peruvian postmenopausal. Gynecol Endocrinol. 2008. 24:470–474.
24. Campbell LV, Redelman MJ, Borkman M, McLay JG, Chisholm DJ. Factors in sexual dysfunction in diabetic female volunteer subjects. Med J Aust. 1989. 151:550–552.
25. Tyrer G, Steel JM, Ewing DJ, Bancroft J, Warner P, Clarke BF. Sexual responsiveness in diabetic women. Diabetologia. 1983. 24:166–171.
26. Pukall CF, Lahaie MA, Binik YM. Goldstein I, Meston CM, Davis SR, Traish AM, editors. 2006 Sexual Pain Disorders: Pathophysiologic Factors. Women's sexual function and dysfunction- study, diagnosis, and treatment. 2006. 1st ed. London: Taylor & Francis;236–242.
27. Kim SW, Kim NN, Jeong SJ, Munarriz R, Goldstein I, Traish AM. Modulation of rat vaginal blood flow and estrogen receptor by estradiol. J Urol. 2004. 172:1538–1543.
28. Kim SW, Jeong SJ, Munarriz R, Kim NN, Goldstein I, Traish AM. An in vivo rat model to investigate female vaginal arousal response. J Urol. 2004. 171:1357–1361.
29. Pessina MA, Hoyt RF Jr, Goldstein I, Traish AM. Differential regulation of the expression of estrogen, progesterone, and androgen receptors by sex steroid hormones in the vagina: immunohistochemical studies. J Sex Med. 2006. 3:804–814.
30. Pessina MA, Hoyt RF Jr, Goldstein I, Traish AM. Differential effects of estradiol, progesterone, and testosterone on vaginal structural integrity. Endocrinology. 2006. 147:61–69.
31. Wells CC, Riazi S, Mankhey RW, Bhatti F, Ecelbarger C, Maric C. Diabetic nephropathy is associated with decreased circulating estradiol levels and imbalance in the expression of renal estrogen receptors. Gend Med. 2005. 2:227–237.
32. Garris DR, Coleman DL, Morgan CR. Age- and diabetes-related changes in tissue glucose uptake and estradiol accumulation in the C57BL/KsJ mouse. Diabetes. 1985. 34:47–52.
33. Garris DR. Diabetes-associated alterations in uterine structure in the C57BL/KsJ mouse: relationship to changes in estradiol accumulation, circulating ovarian steroid levels, and age. Anat Rec. 1985. 211:414–419.
34. Park K, Ryu SB, Park YI, Ahn K, Lee SN, Nam JH. Diabetes mellitus induces vaginal tissue fibrosis by TGF-beta 1 expression in the rat model. J Sex Marital Ther. 2001. 27:577–587.
35. Park K, Ahn K, Chang JS, Lee SE, Ryu SB, Park YI. Diabetes induced alteration of clitoral hemodynamics and structure in the rabbit. J Urol. 2002. 168:1269–1272.
36. Kim NN, Stankovic M, Cushman TT, Goldstein I, Munarriz R, Traish AM. Streptozotocin-induced diabetes in the rat is associated with changes in vaginal hemodynamics, morphology and biochemical markers. BMC Physiol. 2006. 6:4.
37. Garris DR, Garris BL. Hypercytolipidemia promotes diabetes (db/db) mutation-associated utero-ovarian involution: counter-regulatory influences of progesterone. Pathophysiology. 2004. 11:41–50.
38. Garris DR, Garris BL. Estrogenic restoration of functional pancreatic islet cytoarchitecture in diabetes (db/db) mutant C57BL/KsJ mice: relationship to estradiol localization, systemic glycemia, and persistent hyperinsulinemia. Cell Tissue Res. 2005. 319:231–242.
39. Garris DR, Garris BL. Diabetes (db/db) mutation-induced female reproductive tract hypercytolipidemia: estrogenic restoration of utero-ovarian indices. Reprod Toxicol. 2004. 18:641–651.
40. Dixon A, Maric C. 17beta-Estradiol attenuates diabetic kidney disease by regulating extracellular matrix and transforming growth factor-beta protein expression and signaling. Am J Physiol Renal Physiol. 2007. 293:F1678–F1790.
41. Garris DR. Gonadal steroid modulation of the diabetes (db/db) mutation-induced hyperlipometabolic, hypogonadal syndrome: restoration of female reproductive tract cytochemical and structural indices. Pathophysiology. 2005. 12:109–120.
42. Chabrolle C, Jeanpierre E, Tosca L, Ramé C, Dupont J. Effects of high levels of glucose on the steroidogenesis and the expression of adiponectin receptors in rat ovarian cells. Reprod Biol Endocrinol. 2008. 6:11.
43. Stamataki KE, Spina J, Rangou DB, Chlouverakis CS, Piaditis GP. Ovarian function in women with non-insulin dependent diabetes mellitus. Clin Endocrinol (Oxf). 1996. 45:615–621.
44. La Marca A, Morgante G, Palumbo M, Cianci A, Petraglia F, De Leo V. Insulin-lowering treatment reduces aromatase activity in response to follicle-stimulating hormone in women with polycystic ovary syndrome. Fertil Steril. 2002. 78:1234–1239.
45. Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002. 137:25–33.
46. Ferrini MG, Nolazco G, Vernet D, Gonzalez-Cadavid NF, Berman J. Increased vaginal oxidative stress, apoptosis, and inducible nitric oxide synthase in a diabetic rat model: implications for vaginal fibrosis. Fertil Steril. 2006. 86:1152–1163.
47. Cushman T, Kim NN, Hoyt R, Traish A. Estradiol restores diabetes-induced reductions in sex steroid receptor expression and distribution in the vagina of db/db mouse model. J Steroid Biochem Molec Biol. (in press 2009).
48. Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes. 2002. 51:1938–1948.
49. Menini S, Iacobini C, Oddi G, Ricci C, Simonelli P, Fallucca S, et al. Increased glomerular cell (podocyte) apoptosis in rats with streptozotocin-induced diabetes mellitus: role in the development of diabetic glomerular disease. Diabetologia. 2007. 50:2591–2599.
50. Martin PM, Roon P, Van Ells TK, Ganapathy V, Smith SB. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci. 2004. 45:3330–3336.
51. Gao Q, Gao YM. Hyperglycemic condition disturbs the proliferation and cell death of neural progenitors in mouse embryonic spinal cord. Int J Dev Neurosci. 2007. 25:349–357.
52. Gorodeski GI. Estrogen modulation of epithelial permeability in cervical-vaginal cells of premenopausal and postmenopausal women. Menopause. 2007. 14:1012–1019.
53. Park K, Han HJ, Kim SW, Jung SI, Kim SO, Lee HS, et al. Expression of aquaporin water channels in rat vagina: potential role in vaginal lubrication. J Sex Med. 2008. 5:77–82.
54. Akkus E, Carrier S, Baba K, Hsu GL, Padma-Nathan H, Nunes L, et al. Structural alterations in the tunica albuginea of the penis: impact of Peyronie's disease, ageing and impotence. Br J Urol. 1997. 79:47–53.
55. Chin M, Isono M, Isshiki K, Araki S, Sugimoto T, Guo B, et al. Estrogen and raloxifene, a selective estrogen receptor modulator, ameliorate renal damage in db/db mice. Am J Pathol. 2005. 166:1629–1636.
56. Kim NN, Min K, Pessina MA, Munarriz R, Goldstein I, Traish AM. Effects of ovariectomy and steroid hormones on vaginal smooth muscle contractility. Int J Impot Res. 2004. 16:43–50.
57. Cushman T, Kim NN, Hoyt R, Traish A. Estradiol ameliorates diabetes induced changes in vaginal structure of db/db mouse model. J Sex Med. (in press 2009).
58. Giraldi A, Levin RJ. Goldstein I, Meston CM, Davis SR, Traish AM, editors. Vascular Physiology of female sexual function. Women's sexual function and dysfunction-study, diagnosis, and treatment. 2006. 1st ed. London: Taylor & Francis;174–180.
59. Giraldi A, Alm P, Werkström V, Myllymäki L, Wagner G, Andersson KE. Morphological and functional characterization of a rat vaginal smooth muscle sphincter. Int J Impot Res. 2002. 14:271–282.
60. Traish AM, Kim NN, Munarriz R, Goldstein I. Female genital sexual arousal: biochemical mediators and potential mechanisms of dysfunction. Drug Disc Today. 2004. 1:91–97.
61. Giraldi A, Persson K, Werkström V, Alm P, Wagner G, Andersson KE. Effects of diabetes on neurotransmission in rat vaginal smooth muscle. Int J Impot Res. 2001. 13:58–66.
62. El-Sakka AI, Lin CS, Chui RM, Dahiya R, Lue TF. Effects of diabetes on nitric oxide synthase and growth factor genes and protein expression in an animal model. Int J Impot Res. 1999. 11:123–132.
63. Cellek S, Foxwell NA, Moncada S. Two phases of nitrergic neuropathy in streptozotocin-induced diabetic rats. Diabetes. 2003. 52:2353–2362.
64. Caruso S, Rugolo S, Mirabella D, Intelisano G, Di Mari L, Cianci A. Changes in clitoral blood flow in premenopausal women affected by type 1 diabetes after single 100-mg administration of sildenafil. Urology. 2006. 68:161–165.
65. Caruso S, Rugolo S, Agnello C, Intelisano G, Di Mari L, Cianci A. Sildenafil improves sexual functioning in premenopausal women with type 1 diabetes who are affected by sexual arousal disorder: a double-blind, crossover, placebo-controlled pilot study. Fertil Steril. 2006. 85:1496–1501.
66. Resnick HE, Howard BV. Diabetes and cardiovascular disease. Annu Rev Med. 2002. 53:245–267.
67. Masding MG, Stears AJ, Burdge GC, Wootton SA, Sandeman DD. Premenopausal advantages in postprandial lipid metabolism are lost in women with type 2 diabetes. Diabetes Care. 2003. 26:3243–3249.
68. Lee SJ, Lee DW, Kim KS, Lee IK. Effect of estrogen on endothelial dysfunction in postmenopausal women with diabetes. Diabetes Res Clin Pract. 2001. 54:Suppl 2. S81–S92.
69. Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000. 11:319–329.
70. U.S. Renal Data System. USRDS 2000 Annual Data Report. 2000. Bethesda, MD: NIDDK, The National Institutes of Health.
71. Mankhey RW, Bhatti F, Maric C. 17beta-Estradiol replacement improves renal function and pathology associated with diabetic nephropathy. Am J Physiol Renal Physiol. 2005. 288:F399–F405.
72. Rossi R, Origliani G, Modena MG. Transdermal 17-beta-estradiol and risk of developing type 2 diabetes in a population of healthy, nonobese postmenopausal women. Diabetes Care. 2004. 27:645–649.
73. Kernohan AF, Sattar N, Hilditch T, Cleland SJ, Small M, Lumsden MA, et al. Effects of low-dose continuous combined hormone replacement therapy on glucose homeostasis and markers of cardiovascular risk in women with type 2 diabetes. Clin Endocrinol (Oxf). 2007. 66:27–34.
74. Berman JR, McCarthy MM, Kyprianou N. Effect of estrogen withdrawal on nitric oxide synthase expression and apoptosis in the rat vagina. Urology. 1998. 51:650–656.
75. Rosenfeld CR, Chen C, Roy T, Liu X. Estrogen selectively up-regulates eNOS and nNOS in reproductive arteries by transcriptional mechanisms. J Soc Gynecol Investig. 2003. 10:205–215.
76. Ekka E, Vanderheyden I, Glorieux B, de Hertogh R. Estradiol-induced progesterone receptor synthesis in normal and diabetic ovariectomized rat uterus. J Steroid Biochem. 1987. 28:61–64.
77. Leavitt WW, Chen TJ, Allen TC. Regulation of progesterone receptor formation by estrogen action. Ann N Y Acad Sci. 1977. 286:210–225.
78. Kurita T, Lee KJ, Cooke PS, Taylor JA, Lubahn DB, Cunha GR. Paracrine regulation of epithelial progesterone receptor by estradiol in the mouse female reproductive tract. Biol Reprod. 2000. 62:821–830.
79. Pelletier G, Luu-The V, Li S, Labrie F. 2004 localization and estrogenic regulation of androgen receptor mRNA expression in the mouse uterus and vagina. J Endocrinol. 2004. 180:77–85.
80. Traish AM, Kim SW, Stankovic M, Goldstein I, Kim NN. Testosterone increases blood flow and expression of androgen and estrogen receptors in the rat vagina. J Sex Med. 2007. 4:609–619.
81. Traish AM, Kim N, Min K, Munarriz R, Goldstein I. Role of androgens in female genital sexual arousal: Receptor expression, structure, and function. Fertil Steril. 2002. 77:Suppl 4. S11–S18.
82. Alonso A, Fernández R, Moreno M, Ordóñez P, González-Pardo H, Conejo NM, et al. Positive effects of 17beta-estradiol on insulin sensitivity in aged ovariectomized female rats. J Gerontol A Biol Sci Med Sci. 2006. 61:419–426.
83. Andersson B, Mattsson LA, Hahn L, Mårin P, Lapidus L, Holm G, et al. Estrogen replacement therapy decreases hyperandrogenicity and improves glucose homeostasis and plasma lipids in postmenopausal women with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1997. 82:638–643.
84. Araújo DA, Farias ML, Andrade AT. Effects of transdermal and oral estrogen replacement on lipids and glucose metabolism in postmenopausal women with type 2 diabetes mellitus. Climacteric. 2002. 5:286–292.
85. Cagnacci A, Tuveri F, Cirillo R, Setteneri AM, Melis GB, Volpe A. The effect of transdermal 17-beta-estradiol on glucose metabolism of postmenopausal women is evident during the oral but not the intravenous glucose administration. Maturitas. 1997. 28:163–167.
86. Barros RP, Machado UF, Warner M, Gustafsson JA. Muscle GLUT4 regulation by estrogen receptors ERbeta and ERalpha. Proc Natl Acad Sci U S A. 2006. 103:1605–1608.
87. Barros RP, Machado UF, Gustafsson JA. Estrogen receptors: new players in diabetes mellitus. Trends Mol Med. 2006. 12:425–431.
88. Cheng CM, Cohen M, Wang J, Bondy CA. Estrogen augments glucose transporter and IGF1 expression in primate cerebral cortex. FASEB J. 2001. 15:907–915.
89. Mankhey RW, Wells CC, Bhatti F, Maric C. 17beta-estradiol supplementation reduces tubulointerstitial fibrosis by increasing MMP activity in the diabetic kidney. Am J Physiol Regul Integr Comp Physiol. 2007. 292:R769–R777.
90. Shim GJ, Kis LL, Warner M, Gustafsson JA. Autoimmune glomerulonephritis with spontaneous formation of splenic germinal centers in mice lacking the estrogen receptor alpha gene. Proc Natl Acad Sci U S A. 2004. 101:1720–1724.
91. Traish AM, Kim NN, Huang YH, Min K, Munarriz R, Goldstein I. Sex steroid hormones differentially regulate nitric oxide synthase and arginase activities in the proximal and distal rabbit vagina. Int J Impot Res. 2003. 15:397–404.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download