Aggressive angiomyxoma (AAM) usually occurs in the genital and perineal area of female patients and most commonly in the third to fifth decades of life [1]. A retrovesical tumor is defined as "a tumor arising from retrovesical tissue excluding the pelvic organs such as rectum, bladder, prostate, seminal vesicle, vagina or uterus," and may or may not cause lower urinary tract symptoms [2]. This report discusses a rare case of AAM that caused lower urinary tract symptoms and presented itself as a retrovesical tumor on pelvic magnetic resonance imaging (MRI).
CASE REPORT
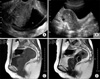
A 66-year-old female patient underwent abdominal pelvis ultrasonography (A-P sono) for a routine check. The A-P sono showed a mass between the urinary bladder and the vagina. For further evaluation of this mass, the patient visited the Department of Urology. She presented with frequency of urination and nocturia of three times per night. Her voiding diary showed that her functional bladder capacity was less than 200 ml. Upon physical examination, her abdomen was soft and movable and there was no tenderness. The mass was palpable through the vagina, and it was soft and round. The laboratory findings were within normal limits and her tumor markers, such as CA 19-9, AFP, and CEA, were within normal ranges. Transvaginal ultrasonography (TVUS) and pelvic MRI were performed. TVUS revealed a 5.1×4.7×3.4 cm sized homogeneous mass that was compressing the urinary bladder (Fig. 1A, B). On the pelvic MRI, the tumor was located in the retrovesical space and it was thought to be arising from the retrovesical tissue, such as the bladder, vagina, or uterus. However, we could not rule out that the tumor might have originated from the urethra. On the T1-weighted image, the tumor was of homogeneous and low intensity, similar to the image of the muscles. On the T2-weighted image, the mass was heterogeneous and of intermediate intensity (Fig. 1C, D). The tumor was thought to be a leiomyoma, a rhabdomyosarcoma, or a neurogenic tumor, as suggested by these MRI findings.
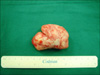
In the operation field, the tumor was adhered to the posterior bladder wall. However, it was easily separated from the bladder wall and was resected en bloc. The resected tumor was 5.5×4×3 cm in size. Grossly, the tumor had a soft, smooth and elastic surface (Fig. 2). The cut surface showed whitish-yellow homogeneous lesions. Microscopically, the mass was made up of rather well-demarcated tumor tissues, which showed low cellularity of relatively uniform, small, satellite and spindly cells that were set in a loosely collagenous, myxoid matrix with scattered vessels of varying caliber. The tumor cells had scant, pale, eosinophilic cytoplasm with poorly defined borders and relatively bland nuclei with open chromatin and a single, small nucleoli. Multinucleated giant cells were rarely observed. Mitotic figures were infrequent. On the basis of these histologic findings, this case was diagnosed as AAM (Fig. 3). Immunohistochemically, the tumor cells showed diffuse staining for vimentin, desmin, estrogen, and progesterone receptors and focal staining for actin (Fig. 4). After the surgery, the patient's nocturia disappeared and her functional bladder capacity increased to 350 ml. At 1 year of follow-up, the patient was free from recurrence.
DISCUSSION
AAM is a rare and benign tumor that involves the connective tissue of the perineal regions in women of reproductive age [1]. Because the patient's age was 66 years old, this case occurred much later than in an average case. In Korea, several cases of AAM have been reported. However, only one case of AAM after menopause has been reported [3].
AAM was first described by Steeper and Rosai in 1983 [4]. They described nine cases of soft tissue tumor in the female genitalia and named it aggressive angiomyxoma. Since then, about 200 cases of this tumor have been reported. Although some male cases have been reported [5], almost all of the reported tumors arose from the perineum in young female patients. Only a few cases of AAM have been discovered in the retrovesical area in older women.
Preoperative diagnosis of tumors in this region is usually based on imaging studies, but such tumors seldom get diagnosed as AAM. The MR features of AAMs have been described as isointense or low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. The voluted pattern of signal intensity on the T2-weighted images is reported as a typical finding in AAMs. The high signal on the T2-weighted MR image is likely to be due to the high water content and myxoid matrix of the tumor [6]. In our case, however, the tumor did not show a low signal intensity on the T1-weighted images and showed intermediate signal intensity on the T2-weighted images.
AAM is derived from myofibroblasts as a phenotypic variant of the basic fibroblast with cytoskeletal adaptation appropriate to situations such as wound healing. The locally invasive character and induction of associated neovascularity are not surprising in a neoplasm of this histogenesis [7].
One of the most important findings for AAM are the immunohistochemical analyses. AAM must be histologically differentiated from other benign and malignant myxoid neoplasms, such as angiofibroblastoma, cutaneous myxoma, myxoid neurofibroma, myxoid leiomyoma, pelvic fibromatosis, myxoid liposarcoma, and low-grade myxoid malignant fibrous histiocytoma. For differential diagnosis, the reactivity for desmin, smooth muscle actin, muscle-specific actin, vimentin, CD34, S100 protein, and estrogen and progestin receptors must be confirmed [1,8]. Reactivity for estrogen and progesterone receptors suggests that this tumor may be a hormone-responsive neoplasm.
According to previous studies, local recurrence of this tumor is common, with a recurrence rate of 50-70%, even if the whole mass is resected en bloc [9]. The tumor was therefore termed "aggressive" angiomyxoma. Most recurrences of AAM have occurred within the first 3 years of the initial diagnosis [10]. However, one case of AAM that recurred 14 years after the en-bloc resection has been reported, so it is necessary to maintain long-term follow-up and to carefully monitor with imaging studies [8]. In our case, the follow-up period has been only 1 year, so it is too early to predict future recurrence.
In conclusion, this case demonstrates that an AAM may occur as a retrovesical tumor and may cause lower urinary tract symptoms. Therefore, AAM should be recognized as a differential diagnosis in a woman presenting with a retrovesical tumor.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download