Abstract
Purpose
To evaluate the efficacy and safety of targeted therapy in patients with metastatic renal cell carcinoma.
Materials and Methods
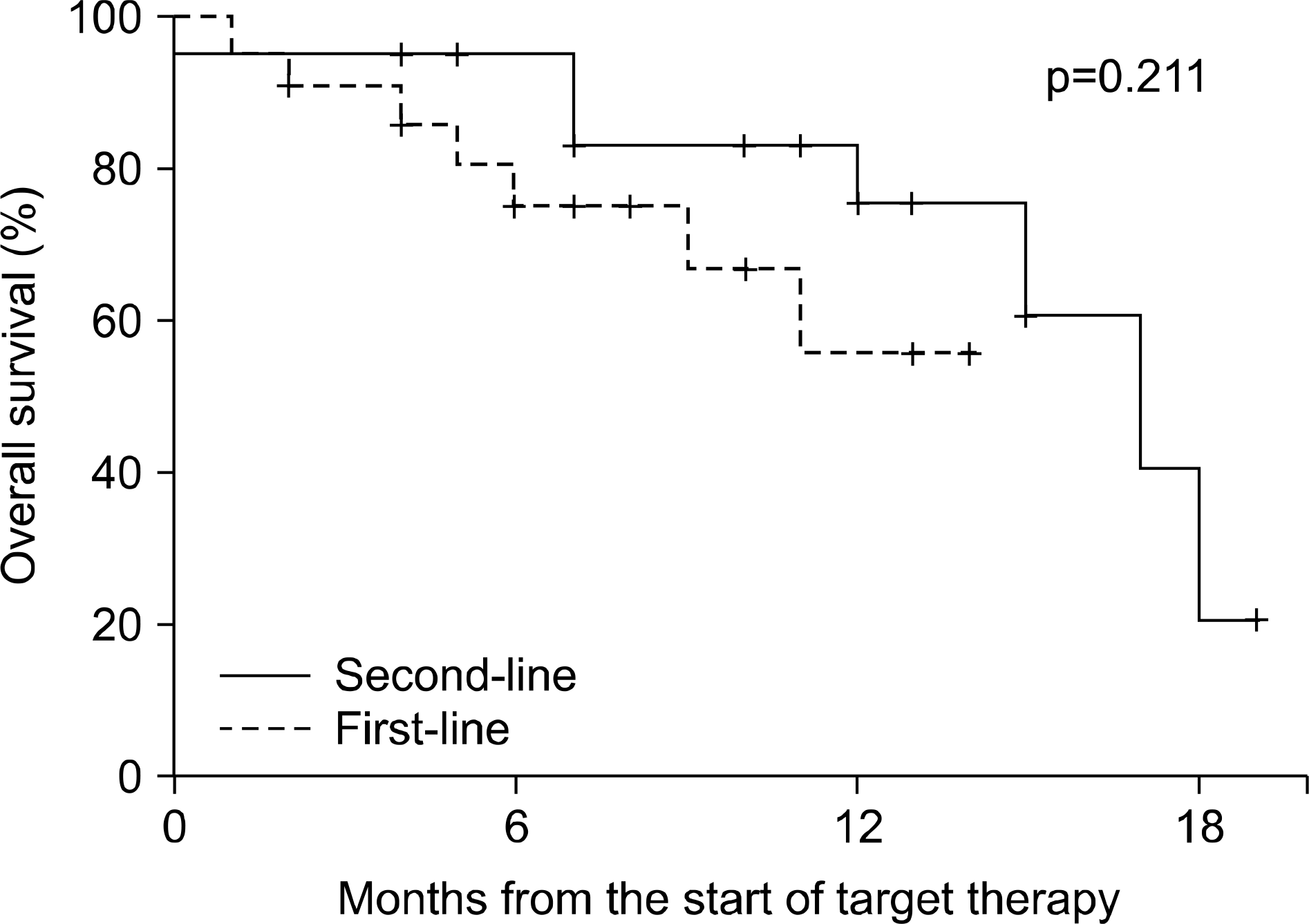
In this retrospective analysis, 43 consecutive patients with metastatic renal cell carcinoma received targeted therapy between December 2005 and December 2007. All patients underwent radical nephrectomy. Twenty-two patients received targeted therapy as a first-line treatment and 21 as a second-line treatment.
Results
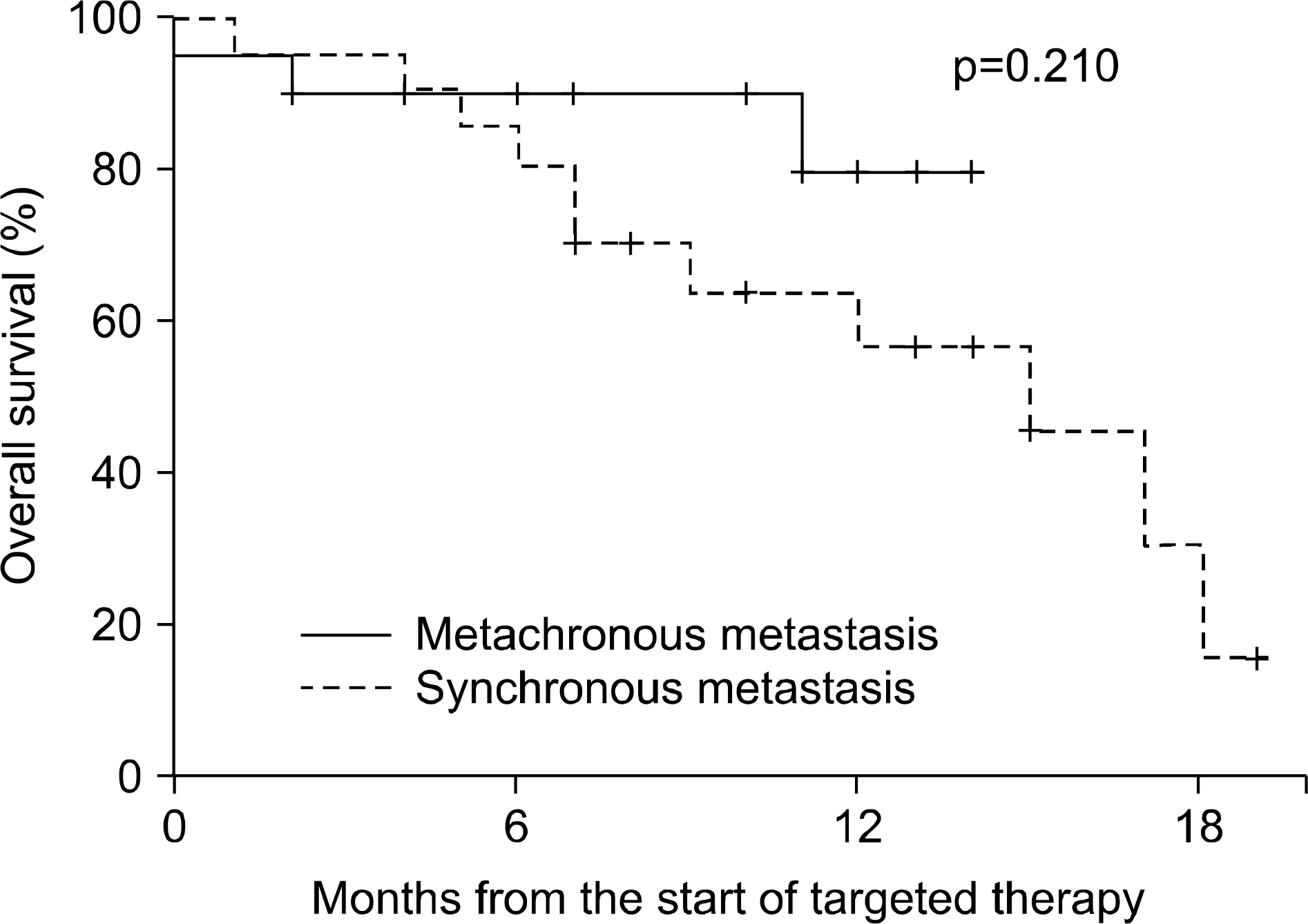
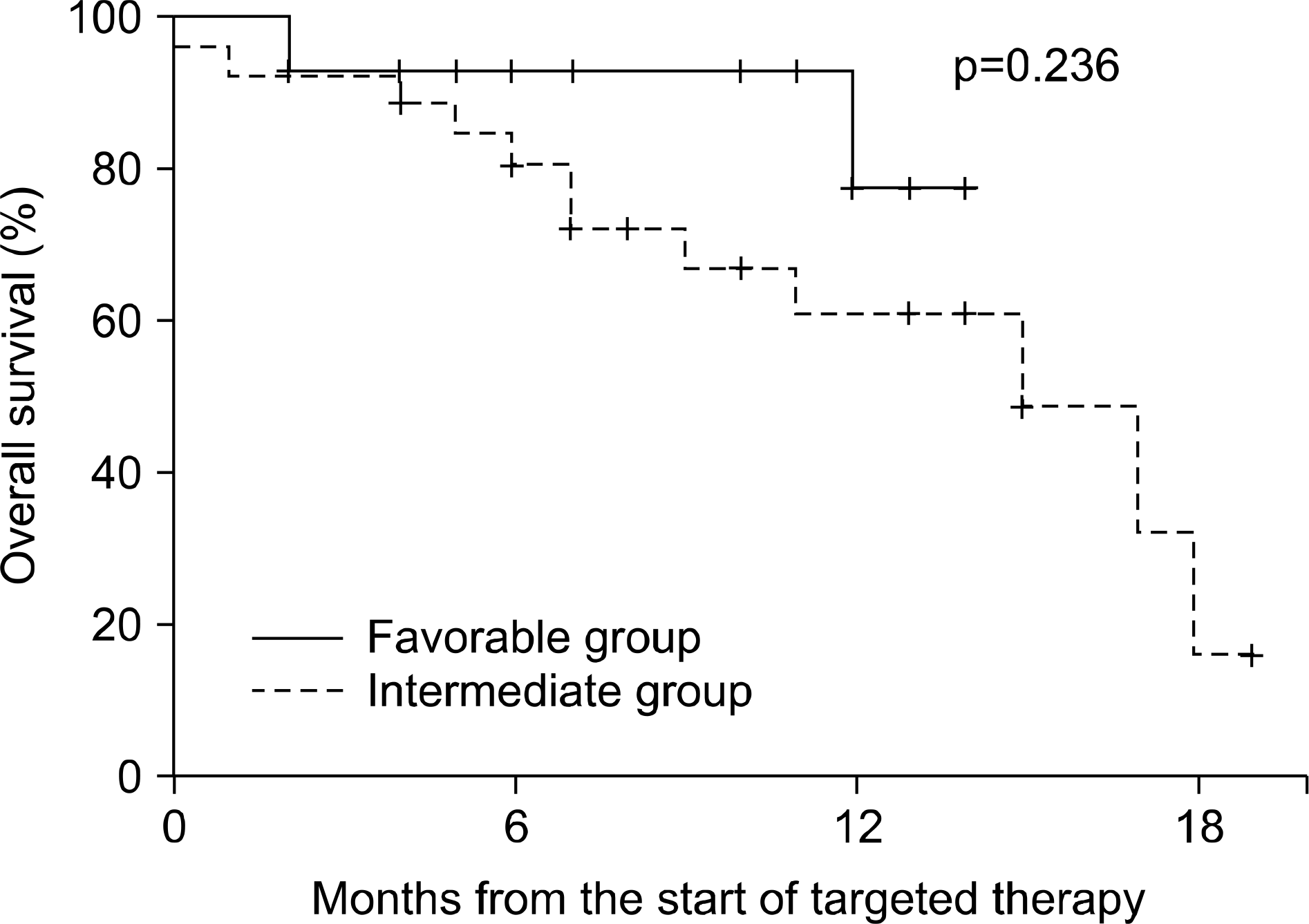
The median follow-up duration after radical nephrectomy and after the initiation of targeted therapy was 87 and 14 months, respectively. The initial response rate was 74.4% (partial response 37.2%, stable disease 37.2%) and the last response rate was 34.9% (partial response 4.7%, stable disease 30.2%). The median survival was 17 months (95% confidence interval (CI), 11.6-22.4) and the median progression-free survival was 10 months (95% CI, 7.5-12.5). Eleven patients (50%) with synchronous metastasis and 3 (14.3%) with metachronous metastasis died from renal cell carcinoma (p=0.023), but there was no significant difference in terms of median survival (15 months vs. longer than 14 months, p=0.210). Also, there was a significant difference in the overall mortality of the MSKCC risk groups (13.3% vs. 44.4%, p=0.049), but no significant difference in median survival (longer than 14 months vs. 15 months, p=0.236).
REFERENCES
1.Jemal A., Siegel R., Ward E., Murray T., Xu J., Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007. 57:43–66.

2.McDermott DF. Update on the application of interleukin-2 in the treatment of renal cell carcinoma. Clin Cancer Res. 2007. 13:716s–20.

3.Pantuck AJ., Zeng G., Belldegrun AS., Figlin RA. Pathobiology, prognosis, and targeted therapy for renal cell carcinoma: exploiting the hypoxia-induced pathway. Clin Cancer Res. 2003. 9:4641–52.
4.Therasse P., Arbuck SG., Eisenhauer EA., Wanders J., Kaplan RS., Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000. 92:205–16.
5.Motzer RJ., Bacik J., Murphy BA., Russo P., Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002. 20:289–96.

6.Motzer RJ., Michaelson MD., Redman BG., Hudes GR., Wilding G., Figlin RA, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006. 24:16–24.

7.Motzer RJ., Rini BI., Bukowski RM., Curti BD., George DJ., Hudes GR, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006. 295:2516–24.

8.Motzer RJ., Hutson TE., Tomczak P., Michalson MD., Bukowski RM., Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007. 356:115–24.

9.Escudier B., Eisen T., Stadler WM., Szczylik C., Oudard S., Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007. 356:125–34.

Fig. 1.
Progression-free survival in 43 patients with metastatic renal cell carcinoma treated with targeted therapy; 14 patients were progression-free at the end of follow-up.
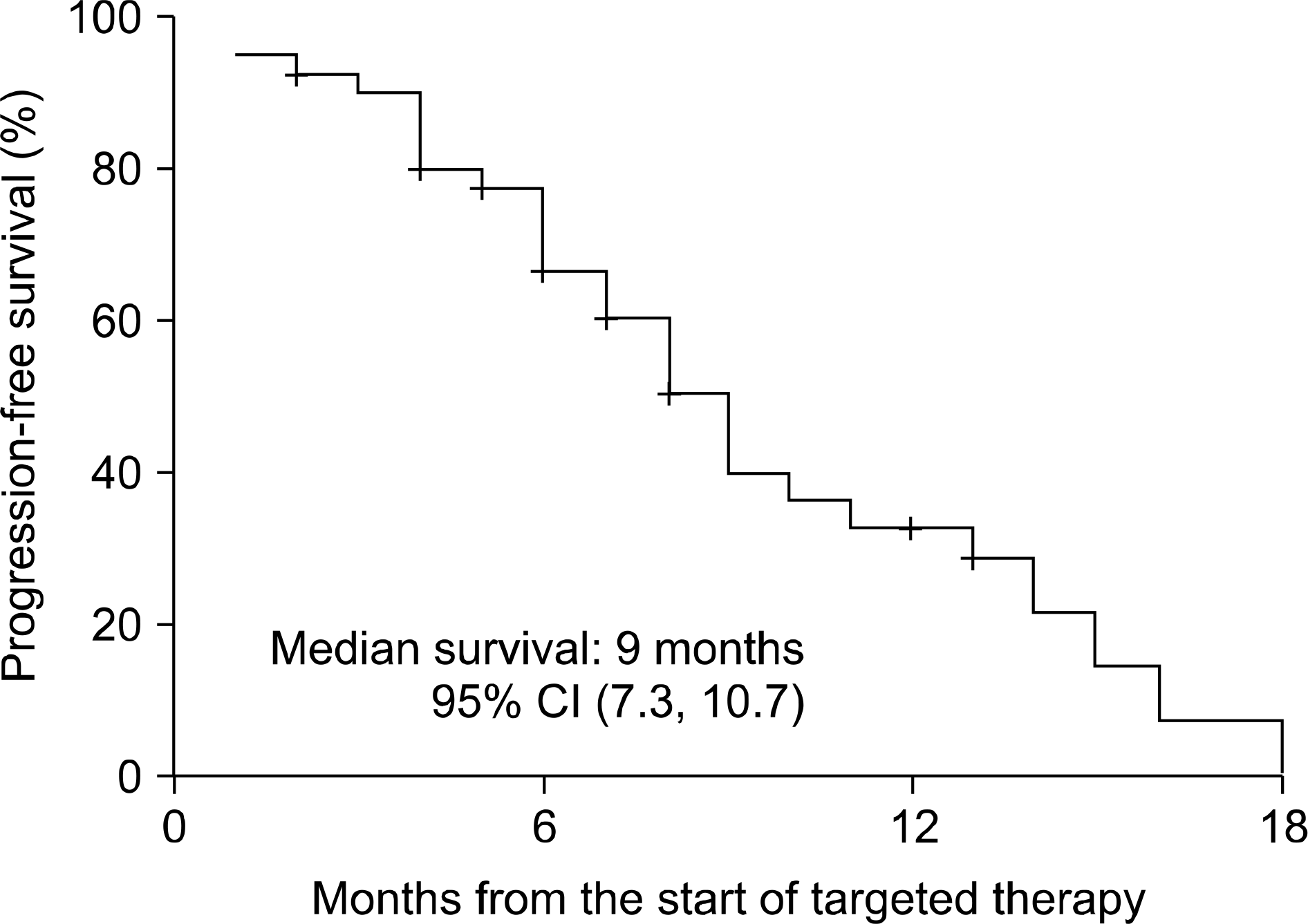
Fig. 2.
Survival time in 43 patients with metastatic renal cell carcinoma treated with targeted therapy; 28 patients were alive at the end of follow-up.
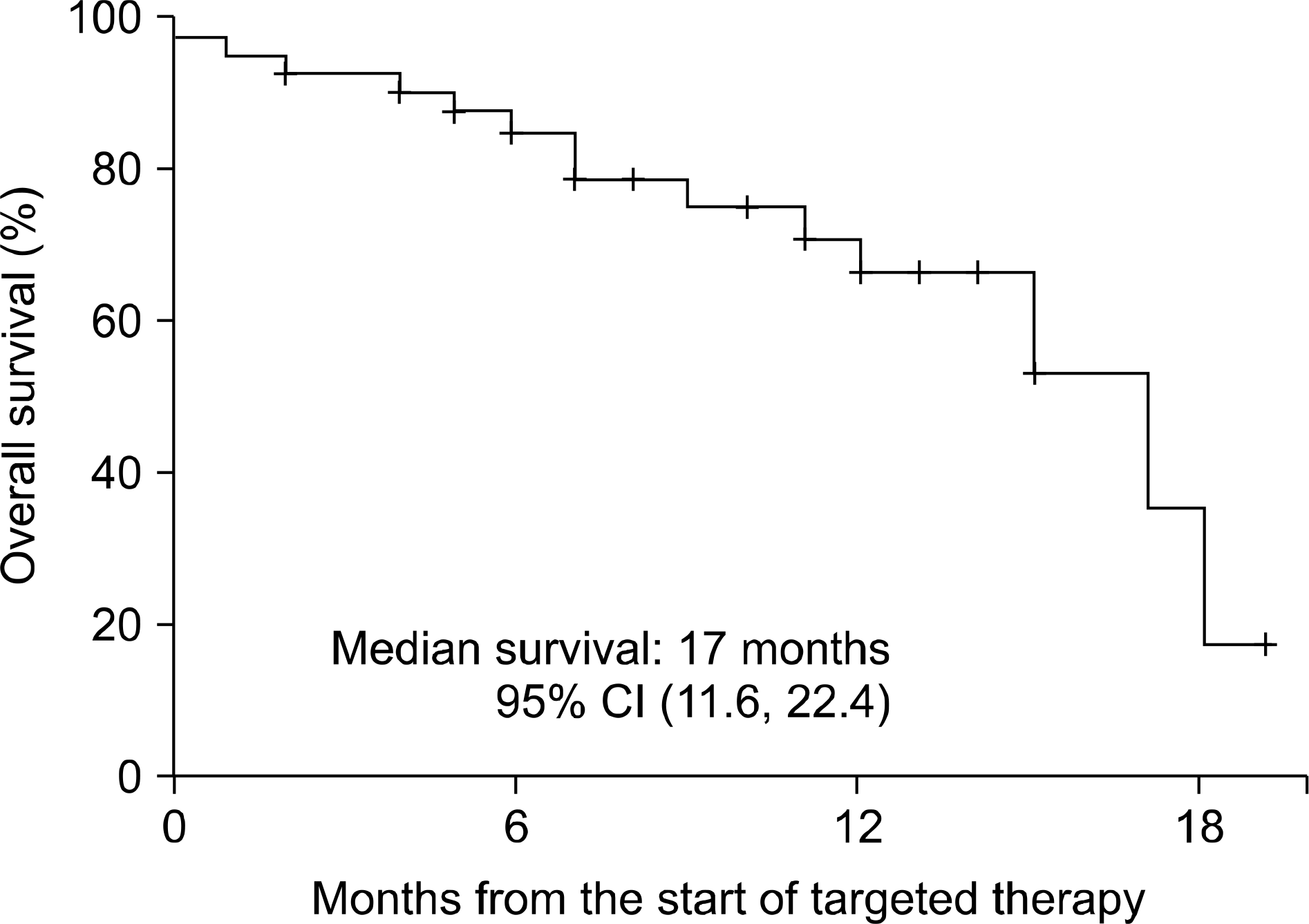
Table 1.
Patient characteristics and best response




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download