Abstract
Purpose
To evaluate the relationship between metabolic syndrome and benign prostatic hyperplasia in Korean men, we investigated the relation between prostate volume and the serum prostate specific-antigen (PSA) level with the factors for metabolic syndrome.
Materials and Methods
We reviewed the data of 1,412 men who had a general health check-up without significant evidence of disease between January 2004 and May 2007. The age, prostate volume, PSA, PSA density and metabolic factors were measured, and the relationships of these factors were evaluated. We also compared the prostate-related data between the metabolic syndrome (MS) group and non-metabolic syndrome (NMS) group.
Results
The prostate volume was significantly larger in the MS group (23.0±7.1ml) than that in the NMS group (20.9±6.1ml) (p<0.001). There was no statistically significant difference of the PSA level between the two groups (MS group: 0.86±0.66, NMS group: 0.90±0.81), but the PSAD was significantly different between the two groups (MS group: 0.038±0.027, NMS group: 0.044±0.031) (p=0.0035). We concluded that there was a significant correlation between the prostate volume and the metabolic syndrome factors. However, when analyzing the influence of each metabolic syndrome factor on the prostate volume, only the BMI was a relatively influential factor.
Conclusions
Our study showed that there was significant correlation between each metabolic syndrome factor and the prostate volume. This seemed to be the result of the commonly related pathophysiology of MS and an enlarged prostrate volume, and obesity was a significant factor. It was meaningful that the PSA level in the MS group was lower than that of the NMS group in case of the same prostate volume.
Go to : 
References
1. Lee C, Kozlowski JM, Grayhack JT. Etiology of benign prostatic hyperplasia. Urol Clin North Am. 1995; 22:237–46.

2. Ziada A, Rosenblum M, Crawford ED. Benign prostatic hyperplasia: an overview. Urology. 1999; 53(3 Suppl 3a):1–6.

3. Hammarsten J, Hogstedt B. Clinical, anthropometric, metabolic and insulin profile of men with fast annual growth rates of benign prostatic hyperplasia. Blood Press. 1999; 8:29–36.
4. Matsuda T, Abe H, Suda K. Relation between benign prostatic hyperplasia and obesity and estrogen. Rinsho Byori. 2004; 52:291–4.
5. Kim JH, Shim BS, Kim JS, Hong YS. Voiding dysfunction of men is associated with metabolic syndrome. Korean J Urol. 2006; 47:257–62.

6. Sohn JC, Chang HS, Kim CI. The correlation between metabolic syndrome and the prostate volume. Korean J Urol. 2007; 48:603–7.

7. Collins GN, Lee RJ, McKelvie GB, Rogers AN, Hehir M. Relationship between prostate specific antigen, prostate volume and age in the benign prostate. Br J Urol. 1993; 71:445–50.

8. Lee SE, Kim DY, Kwak C. Interrelationship among age, prostate specific antigen and prostate volume in Korean men living at the metropolitan area. Korean J Urol. 1999; 40:1311–7.
9. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002; 287:356–9.

10. Lym YL, Hwang SW, Shim HJ, Oh EH, Chang YS, Cho BL. Prevalence and risk factors of the metabolic syndrome as defined by NCEP-APT III. J Korean Acad Fam Med. 2003; 24:135–43.
11. Lim S, Park KS, Lee HK, Cho SI. Changes in the characteristics of metabolic syndrome in Korea over the period 1998–2001 as determined by Korean National Health and Nutrition Examination Surveys. Diabetes Care. 2005; 28:1810–2.

12. Dahle SE, Chokkalingam AP, Gao YT, Deng J, Stanczyk FZ, Hsing AW. Body size and serum levels of insulin and leptin in relation to the risk of benign prostatic hyperplasia. J Urol. 2002; 168:599–604.

13. Hammarsten J, Hogstedt B, Holthuis N, Mellstrom D. Components of the metabolic syndrome-risk factors for the development of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 1998; 1:157–62.

14. McNeal J. Pathology of benign prostatic hyperplasia. Insight into etiology. Urol Clin North Am. 1990; 17:477–86.
15. Michel MC, Mehlburger L, Schumacher H, Bressel HU, Goepel M. Effect of diabetes on lower urinary tract symptoms in patients with benign prostatic hyperplasia. J Urol. 2000; 163:1725–9.

16. Shieh S, Sheu WH, Shen DC, Fuh MM, Chen YD, Reaven GM. Glucose, insulin, and lipid metabolism in doxazosin-treated patients with hypertension. Am J Hypertens. 1992; 5:827–31.

17. Nilsson PM, Lindholm LH, Schersten BF. Life style changes improve insulin resistance in hyperinsulinaemic subjects: a one-year intervention study of hypertensives and normoten-sives in Dalby. J Hypertens. 1992; 10:1071–8.
18. Sugaya K, Kadekawa K, Ikeharama A, Nakayama T, Gakiya M, Nashiro F, et al. Influence of hypertension on lower urinary tract symptoms in benign prostatic hyperplasia. Int J Urol. 2003; 10:569–74.

19. Ozden C, Ozdal OL, Urganciogu G, Koyuncu H, Gokkaya S, Memis A. The correlation between metabolic syndrome and prostatic growth in patients with benign prostatic hyperplasia. Eur Urol. 2007; 51:199–203.

20. Seitter WR, Barrett-Connor E. Cigarette smoking, obesity, and benign prostatic hypertrophy: a prospective population-based study. Am J Epidemiol. 1992; 135:500–3.

21. Gupta A, Gupta S, Pavuk M, Roehrborn CG. Anthropometric and metabolic factors and risk of benign prostatic hyperplasia: a prospective cohort study of Air Force veterans. Urology. 2006; 68:1198–205.

22. Gann PH, Hennekens CH, Longcope C, Verhoek-Oftedahl W, Grodstein F, Stampfer MJ. A prospective study of plasma hormone levels, nonhormonal factors, and development of of benign prostatic hyperplasia. Prostate. 1995; 26:40–9.
23. Kwon NS, Jo MK, Park K. The relationship between the metabolic syndrome and the risk of benign prostatic hyperplasia: a hospital-based study from a health screening population. Korean J Urol. 2007; 48:1016–21.

24. Kim YD, Yang WJ, Song YS, Park YH, Correlation between prostate volume and metabolic or anthropometric factors in male visitors to a health promotion center. Korean J Urol. 2008; 49:139–44.
25. Chung BH, Hong SJ, Lee SE, Lee DH. Serum PSA concentration, prostate specific antigen density with aging. Korean J Urol. 1996; 37:257–62.
26. Roehrborn CG, McConnell J, Bonilla J, Rosenblatt S, Hudson PB, Malek GH, et al. Serum prostate specific antigen is a strong predictor of future growth in men with benign prostatic hyperplasia. PROSCAR longterm efficacy and safety study. J Urol. 2000; 163:13–20.
27. Freedland SJ, Platz EA, Presiti JC Jr, Aronson WJ, Amling CL, Kane CJ, et al. Obesity, serum prostate specific antigen and prostate size: implications for prostate cancer detection. J Urol. 2006; 175:500–4.

28. Baillargeon J, Pollock BH, Kristal AR, Bradshaw P, Hernandez J, Basler J, et al, The association of body mass index and prostate-specific antigen in a population-based study. Cancer. 2005; 103:1092–5.
Go to : 
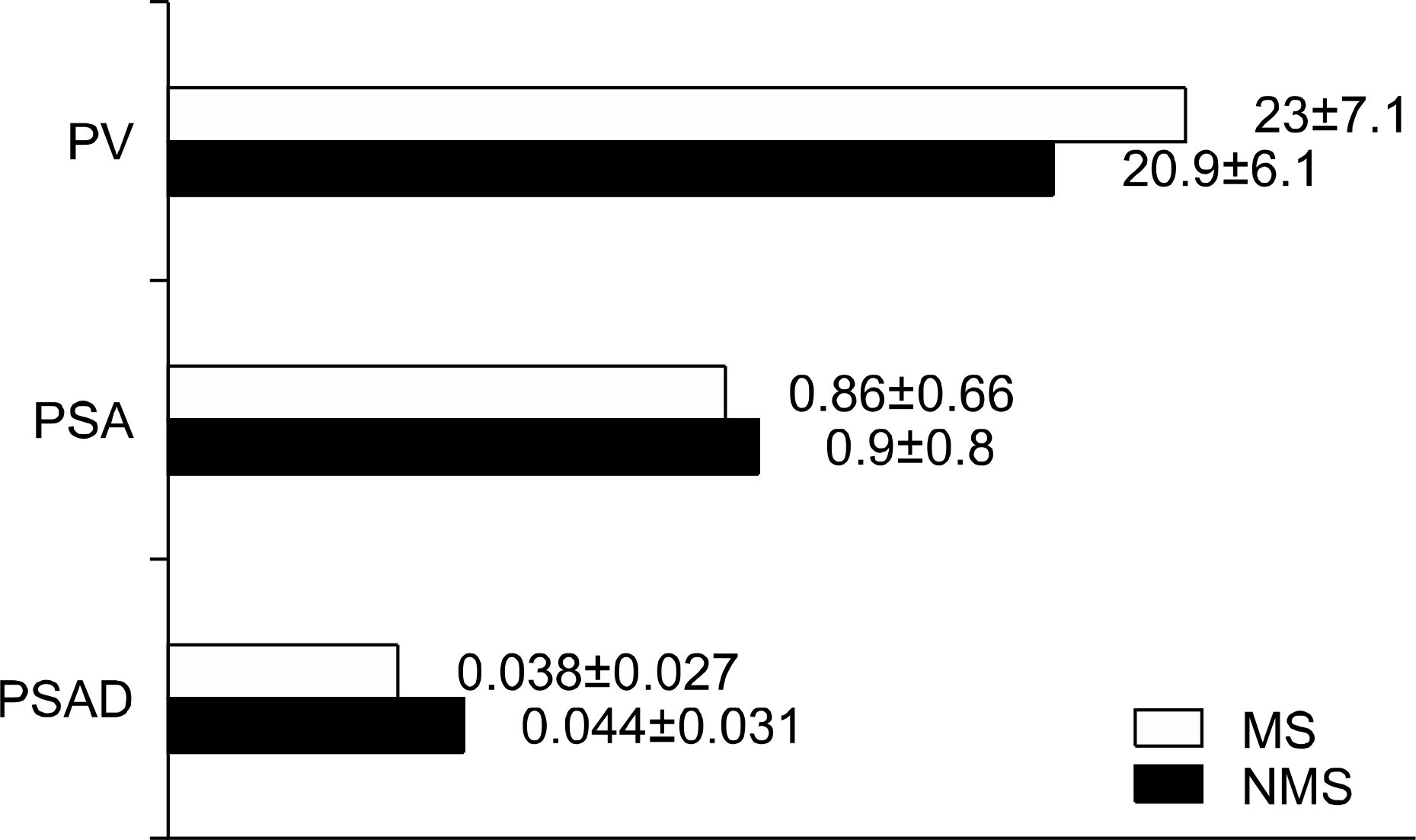 | Fig. 1.The PV, PSA and PSAD between the MS group and the NMS group. PV: prostate volume, PSA: prostate-specific antigen, PSAD: prostate-specific antigen density, MS: metabolic syndrome, NMS: non-metabolic syndrome. |
Table 1.
Comparison of the factors between the MS group and the NMS group
MS: metabolic syndrome, NMS: non-metabolic syndrome, SD: standard deviation, PV: prostate volume, PSA: prostate-specific antigen, PSAD: prostate-specific antigen density, SBP: systolic blood pressure, DBP: diastolic blood pressure, FBS: fasting blood sugar, BMI: body mass index, TG: triglyceride, HDL: high density lipoprotein




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download