Abstract
Purpose
We investigated epidermal growth factor receptor (EGFR) expression in prostate cancer (PCa) and their potential role as predicting factor on biochemical recurrence (BCR).
Materials and Methods
Between February 2005 and February 2007, EGFR expression were prospectively evaluated in a consecutive series of 88 PCa patients with the following characteristics: 66 patients treated with retropubic radical prostatectomy (RRP); 22 patients treated with neoadjuvant hormonal therapy followed by RRP. The relationship between EGFR expression and several clinicopathologic parameters were evaluated. The probability of BCR-free survival was determined using the Kaplan-Meier method.
Results
EGFR expression, was evaluated by immunohistochemistry, was found in 31 of 88 (35.2%) patients. 8 of 31 EGFR-positive patients (25.8%) had BCR, whereas only 5 of 57 EGFR-negative patients (8.8%) had BCR (p=0.031) during a median follow-up of 21 months. Among several variables, high serum prostate-specific antigen values (≥20), extraprostatic extension, seminal vesicle invasion, and EGFR expression were the significant predictors of BCR on univariate analysis. But, multivariate analysis showed that no variable was significant predictor of BCR. EGFR-negative patients had a significantly longer mean BCR-free survival time than EGFR-positive patients (p=0.027).
Go to : 
References
1. Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998; 90:766–71.

2. Kattan MW, Wheeler TM, Scardino PT. Postoperative nomogram for disease recurrence after radical prostatectomy for prostate cancer. J Clin Oncol. 1999; 17:1499–507.

3. Hong JH, Lee HM, Choi HY. The predictors of biochemical recurrence and metastasis following radical perineal prostatectomy in clinically localized prostate cancer. Korean J Urol. 2005; 46:1161–7.
4. Arteaga CL. Epidermal growth factor receptor dependence in human tumors: more than just expression? Oncologist. 2002; 7(Suppl 4):31–9.

5. Di Lorenzo G, Tortora G, D'Armiento FP, De Rosa G, Staibano S, Autorino R, et al. Expression of epidermal growth factor receptor correlates with disease relapse and progression to androgen-independence in human prostate cancer. Clin Cancer Res. 2002; 8:3438–44.
6. Rocha-Lima CM, Soares HP, Raez LE, Singal R. EGFR targeting of solid tumors. Cancer Control. 2007; 14:295–304.

7. Herbst RS, Shin DM. Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: a new paradigm for cancer therapy. Cancer. 2002; 94:1593–611.
8. Merlino GT. Epidermal growth factor receptor regulation and function. Semin Cancer Biol. 1990; 1:277–84.
9. Haeder M, Rotsch M, Bepler G, Henning C, Havemann K, Heimann B, et al. Epidermal growth factor receptor expression in human lung cancer cell lines. Cancer Res. 1988; 48:1132–6.
10. Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001; 2:127–37.

11. Boonstra J, De Laat SW, Ponec M. Epidermal growth factor receptor expression related to differentiation capacity in normal and transformed keratinocytes. Exp Cell Res. 1985; 161:421–33.

12. Greenberg SD, Fraire AE, Kinner BM, Johnson EH. Tumor cell type versus staging in the prognosis of carcinoma of the lung. Pathol Annu. 1987; 22:387–405.
13. Newby JC, Johnston SR, Smith IE, Dowsett M. Expression of epidermal growth factor receptor and c-erbB2 during the development of tamoxifen resistance in human breast cancer. Clin Cancer Res. 1997; 3:1643–51.
14. Chen X, Yeung TK, Wang Z. Enhanced drug resistance in cells coexpressing ErbB2 with EGF receptor or ErbB3. Biochem Biophys Res Commun. 2000; 277:757–63.

15. Fischer-Colbrie J, Witt A, Heinzl H, Speiser P, Czerwenka K, Sevelda P, et al. EGFR and steroid receptors in ovarian carcinoma: comparison with prognostic parameters and outcome of patients. Anticancer Res. 1997; 17:613–9.
16. Magne N, Pivot X, Bensadoun RJ, Guardiola E, Poissonnet G, Dassonville O, et al. The relationship of epidermal growth factor receptor levels to the prognosis of unresectable pharyngeal cancer patients treated by chemoradiotherapy. Eur J Cancer. 2001; 37:2169–77.
17. Mckenzie SJ. Diagnostic utility of oncogenes and their products in human cancer. Biochem Biophys Acta. 1991; 1072:193–214.

18. Culig Z, Hobisch A, Cronauer MV, Radmayr C, Hittmair A, Zhang J, et al. Regulation of prostatic growth and function by peptide growth factors. Prostate. 1996; 28:392–405.

19. Hiramatsu M, Kashimata M, Minami N, Sato A, Murayama M, Minami N. Androgenic regulation of epidermal growth factor in the mouse ventral prostate. Biochem Int. 1998; 17:311–7.
20. Nishi N, Oya H, Matsumoto K, Nakamura T, Miyanaka H, Wada F. Changes in gene expression of growth factors and their receptors during castration-induced involution and androgen-induced regrowth of rat prostate. Prostate. 1996; 28:139–52.
21. Fowler JE Jr, Lau JL, Ghosh L, Mills SE, Mounzer A. Epidermal growth factor and prostatic carcinoma: an immunohistochemical study. J Urol. 1988; 139:857–61.

22. Yang Y, Chisholm GD, Habib FK. Epidermal growth factor and transforming growth factor alpha concentrations in BPH and cancer of the prostate: their relationship with tissue androgen levels. Br J Cancer. 1993; 67:152–5.
23. Robertson CN, Roberson KM, Herzberg AJ, Kerns BJ, Dodge RK, Paulson DF. Differential immunoreactivity of transforming growth factor alpha in benign, dysplastic and malignant prostatic tissues. Surg Oncol. 1994; 3:237–42.

24. Fox SB, Persad RA, Coleman N, Day CA, Silcocks PB, Collins CC. Prognostic value of c-erbB-2 and epidermal growth factor receptor in stage A1 (T1a) prostatic adenocarcinoma. Br J Urol. 1994; 74:214–20.
25. MacDonald A, Habib FK. Divergent responses to epidermal growth factor in hormone sensitive and insensitive human prostate cancer cell lines. Br J Cancer. 1992; 65:177–82.

26. Scher HI, Sarkis A, Reuter V, Cohen D, Netto G, Petrylak D, et al. Changing pattern of expression of the epidermal growth factor receptor and transforming growth factor alpha in the progression of prostatic neoplasms. Clin Cancer Res. 1995; 1:545–50.
27. Barton J, Blackledge G, Wakeling A. Growth factors and their receptors: new targets for prostate cancer therapy. Urology. 2001; 58(2 Suppl 1):114–22.

28. Canil CM, Moore MJ, Winquist E, Baetz T, Pollak M, Chi KN, et al. Randomized phase II study of two doses of gefitinib in hormone-refractory prostate cancer: a trial of the National Cancer Institute of Canada-Clinical Trials Group. J Clin Oncol. 2005; 23:455–60.

29. Miller K, Raabe N, Trachtenberg J, Trump D, Wilding G, Das-Gupta A. ZD1839, an epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), is well tolerated in combination with mitoxantrone and prednisone in patients with hormone-refractory prostate cancer (HRPC). Ann Oncol. 2002; 13(Suppl 5):91. abstract 327.
30. Soulie P, Trump D, Wilding G, Small E, Das-Gupta A. ZD1839, an epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), in combination with docetaxel and estramustine in patients with hormone-refractory prostate cancer (HRPC). Ann Oncol. 2002; 13(Suppl 5):91. abstract 328.
Go to : 
 | Fig. 1.H&E and immunohistochemical evaluation of epidermal growth factor receptor (EGFR) expression in prostate cancer. (A) EGFR-positive tumor (blackish stained) (x200), (B) EGFR-negative tumor (x200). |
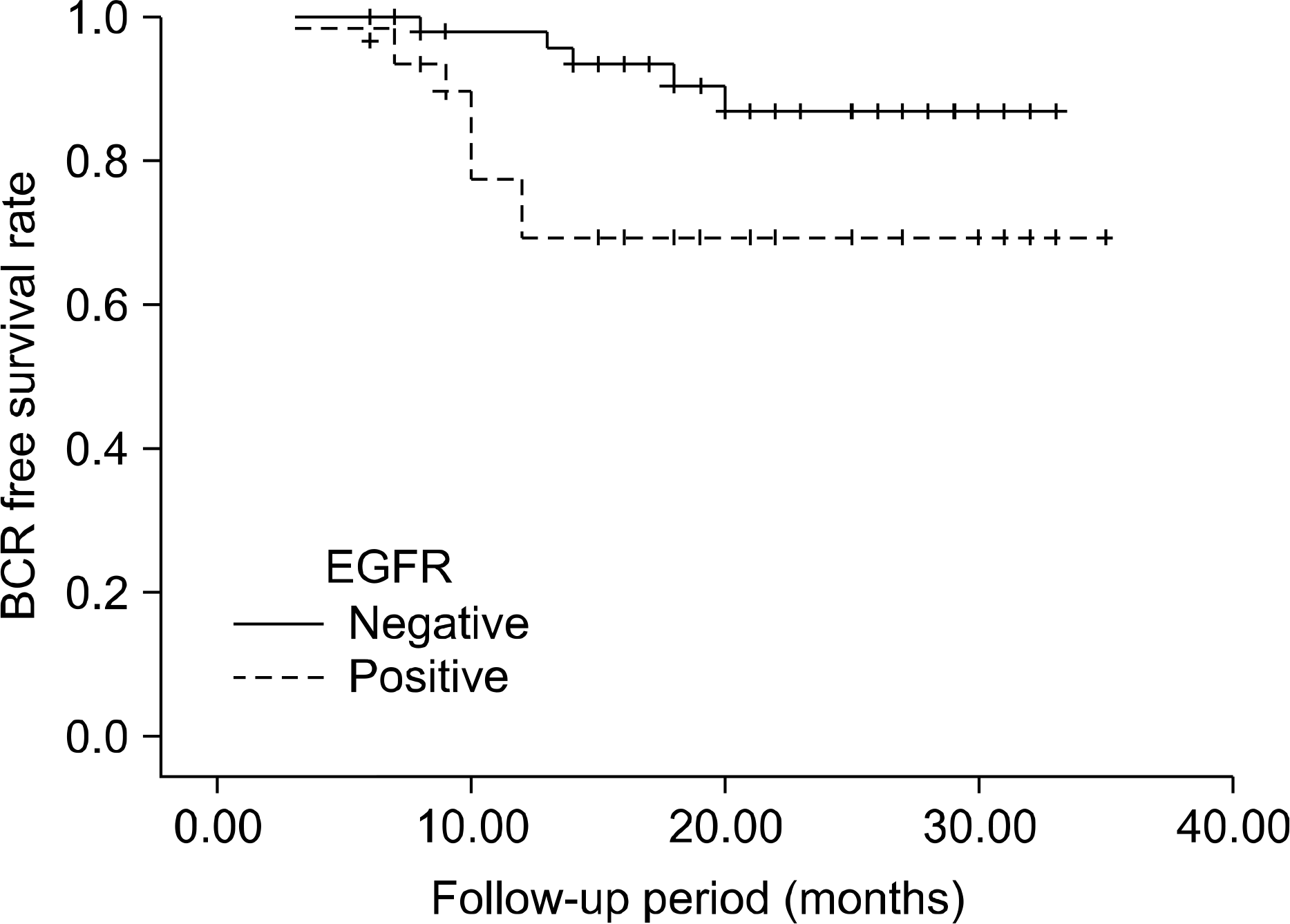 | Fig. 2.Kaplan-Meier method estimates of likelihood of remaining free of biochemical recurrence as related to epidermal growth factor receptor expression (log rank, p=0.027). BCR: biochemical recurrence, EGFR: epidermal growth factor receptor. |
Table 1.
Patient characteristics
Table 2.
Correlation between immunohistochemistry staining for EGFR and clinicopathologic factors
Table 3.
Univariate and multivariate analysis of prognostic factors for biochemical recurrence




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download