Abstract
Purpose
This investigation was undertaken to determine the damage to the testes and epididymides following torsion and detorsion of the testes.
Materials and Methods
The right testes of 8-week-old male rats (n=30) were subjected to torsion for 10 min. At 0, 1, 4, 8, and 24 hours, and 1 week after the repair of a torsion, the ipsilateral and contralateral testes and epididymides were harvested. The mean number of spermatids per tubule, the mean seminiferous tubular diameter (MSTD), and the germinal epithelial cell thickness (GECT) were used to evaluate changes to the testes. The histologic changes to the epididymal ductal epithelium were also evaluated.
Results
The mean number of spermatids per tubule, GECT, and MSTD were significantly decreased in the 24-hour ipsilateral detorsion group, but minimal changes to ipsilateral testes were observed in the 1-week detorsion group. There was no evidence of histologic changes to the testes in any of the contralateral detorsion groups. The interstitial fibroblast proliferation and hemorrhage of the ipsilateral epididymis were found in the 4-hour detorsion group and increased in the 8-hour detorsion group. Interstitial fibroblast proliferation was prominent in the ipsilateral epididymis of the 24-hour detorsion group, but was only occasionally observed in the contralateral epididymides. Shortening of the tubular epithelial cell height and tubule dilatation were observed in the ipsilateral and contralateral epididymis 1 week after detorsion.
Go to : 
References
1. Anderson JB, Williamson RC. The fate of the human testes following unilateral torsion of the spermatic cord. Br J Urol. 1986; 58:698–704.

2. Park EC, Kwak C, Son H, Oh SJ, Lee SE, Choi H. The role of clusterin in apoptosis induced by testicular torsion. Korean J Urol. 2004; 45:380–9.
3. Oh SJ, Park CS, Lee KH, Kim DJ, Lim D, Jie JR, et al. Alteration of nitric oxide synthase subtype expression in contralateral testis of the rat in response to unilateral testicular torsion followed by detorsion. Korean J Urol. 2000; 41:650–8.
5. Hinton BT, Palladino MA. Epididymal epithelium: its contribution to the formation of a luminal fluid microenvironment. Microsc Res Tech. 1995; 30:67–81.

6. Tunckiran A, Cayan S, Bozlu M, Yilmaz N, Acar D, Akbay E. Protective effect of vascular endothelial growth factor on histologic changes in testicular ischemia-reperfusion injury. Fertil Steril. 2005; 84:468–73.
7. Thomas WE, Cooper MJ, Crane GA, Lee G, Williamson RC. Testicular exocrine malfunction after torsion. Lancet. 1984; 2:1357–60.

8. Krarup T. The testis after torsion. Br J Urol. 1978; 50:43–6.
9. Bartsch G, Frank S, Marberger H, Mikuz G. Testicular torsion: late results with special regard to fertility and endocrine function. J Urol. 1980; 124:375–8.

10. Nagler HM, White RD. The effect of testicular torsion on the contralateral testis. J Urol. 1982; 128:1343–8.

11. Kizilcan F, Bernay I, Tanyel FC, Buyukpamukcu N, Bekdik C, Hicsonmez A. Ipsilateral and contralateral testicular blood flows during unilateral testicular torsion by 133Xe clearance technique. Int Urol Nephrol. 1992; 24:515–20.
12. Salman AB, Mutlu S, Iskit AB, Guc MO, Mutlu M, Tanyel FC. Hemodynamic monitoring of the contralateral testis during unilateral testicular torsion describes the mechanism of damage. Eur Urol. 1998; 33:576–80.

13. Vega M, Urrutia L, Iniguez G, Gabler F, Devoto L, Johnson MC. Nitric oxide induces apoptosis in the human corpus luteum in vitro. Mol Hum Reprod. 2000; 6:681–7.

14. Hadziselimovic F, Geneto R, Emmons LR. Increased apoptosis in the contralateral testes of patients with testicular torsion as a factor for infertility. J Urol. 1998; 160:1158–60.

15. Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci U S A. 2005; 102:16696–700.

16. Itoh M, Miyamoto K, Ohga T, Takeuchi Y. Spontaneous occurrence of vasculitis-like lesions in male reproductive tissues in mice: a histological study. Arch Androl. 1999; 42:151–9.
17. Head JR, Billingham RE. Immune privilege in the testis. II. Evaluation of potential local factors. Transplantation. 1985; 40:269–75.
Go to : 
 | Fig. 1.Photomicrograph of unilateral and contralateral testes after testicular torsion and repair of the torsion. (A) Ipsilateral rat testis 0 hours after detorsion, demonstrating a normal seminiferous tubular contour. (B) Contralateral rat testis 0 hours after detorsion, demonstrating a normal seminiferous tubular contour. (C) Ipsilateral rat testis 8 hours after detorsion, demonstrating some interstitial congestion and slight shortening of the germinal epithelial cell layer. (D) Contralateral rat testis 8 hours after detorsion, demonstrating a normal seminiferous tubular contour. (E) Ipsilateral rat testis 24 hours after detorsion, demonstrating more increased shortening of the germinal epithelial cell layer and a decreased number of spermatids in the seminiferous tubule. (F) Contralateral rat testis 24 hours after detorsion, demonstrating a normal seminiferous tubular contour. (G) Ipsilateral rat testis 1 week after detorsion, demonstrating a normal seminiferous tubular contour. (H) Contralateral rat testis 1 week after detorsion, demonstrating a normal seminiferous tubular contour (H&E, x200). |
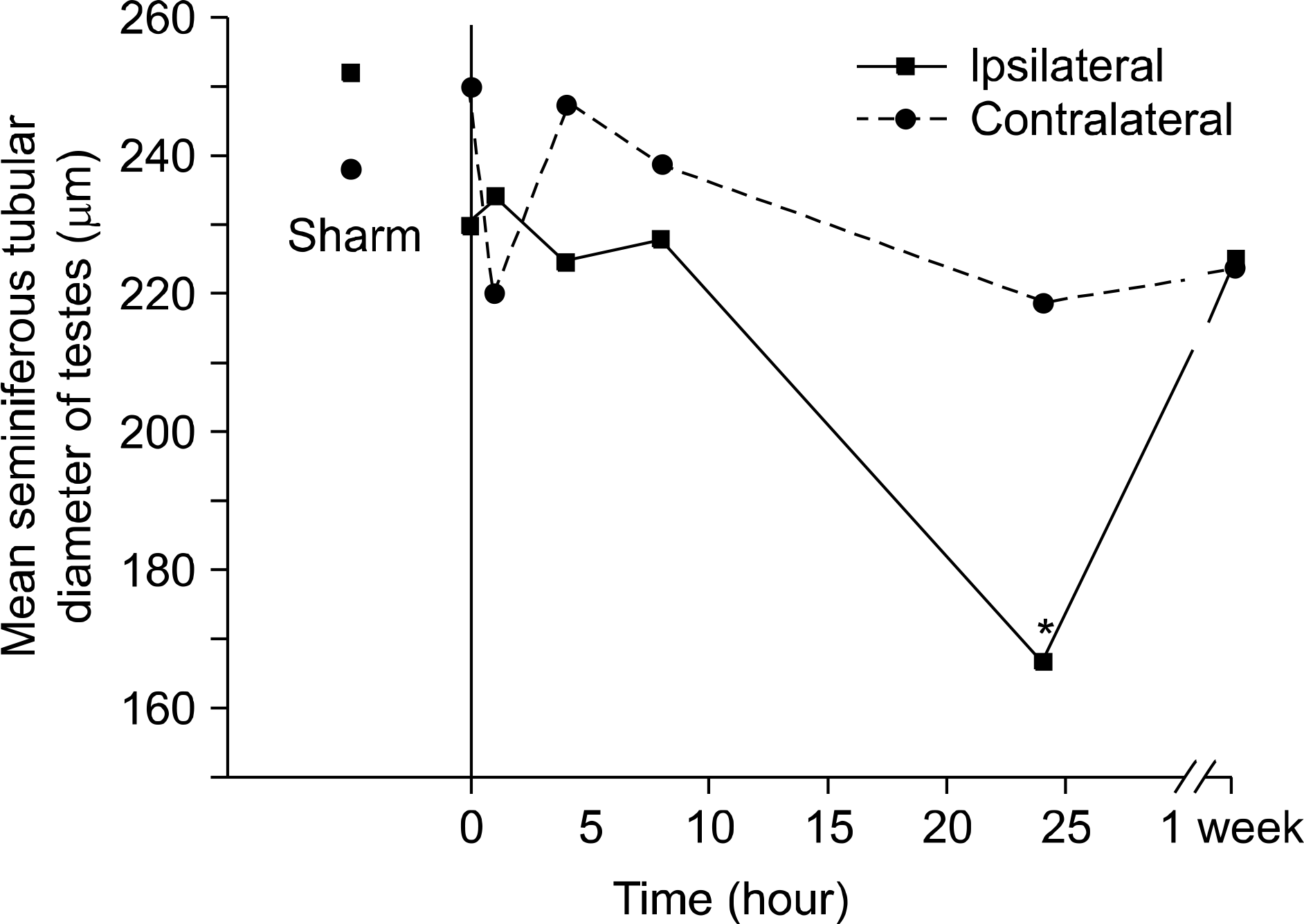 | Fig. 2.The mean seminiferous tubular diameter of the ipsilateral testes taken 24 hours after repair of the torsion was significantly decreased. Asterisk indicated p<0.05, multiple comparison (Dunn procedure) with Kruskall-Wallis test. |
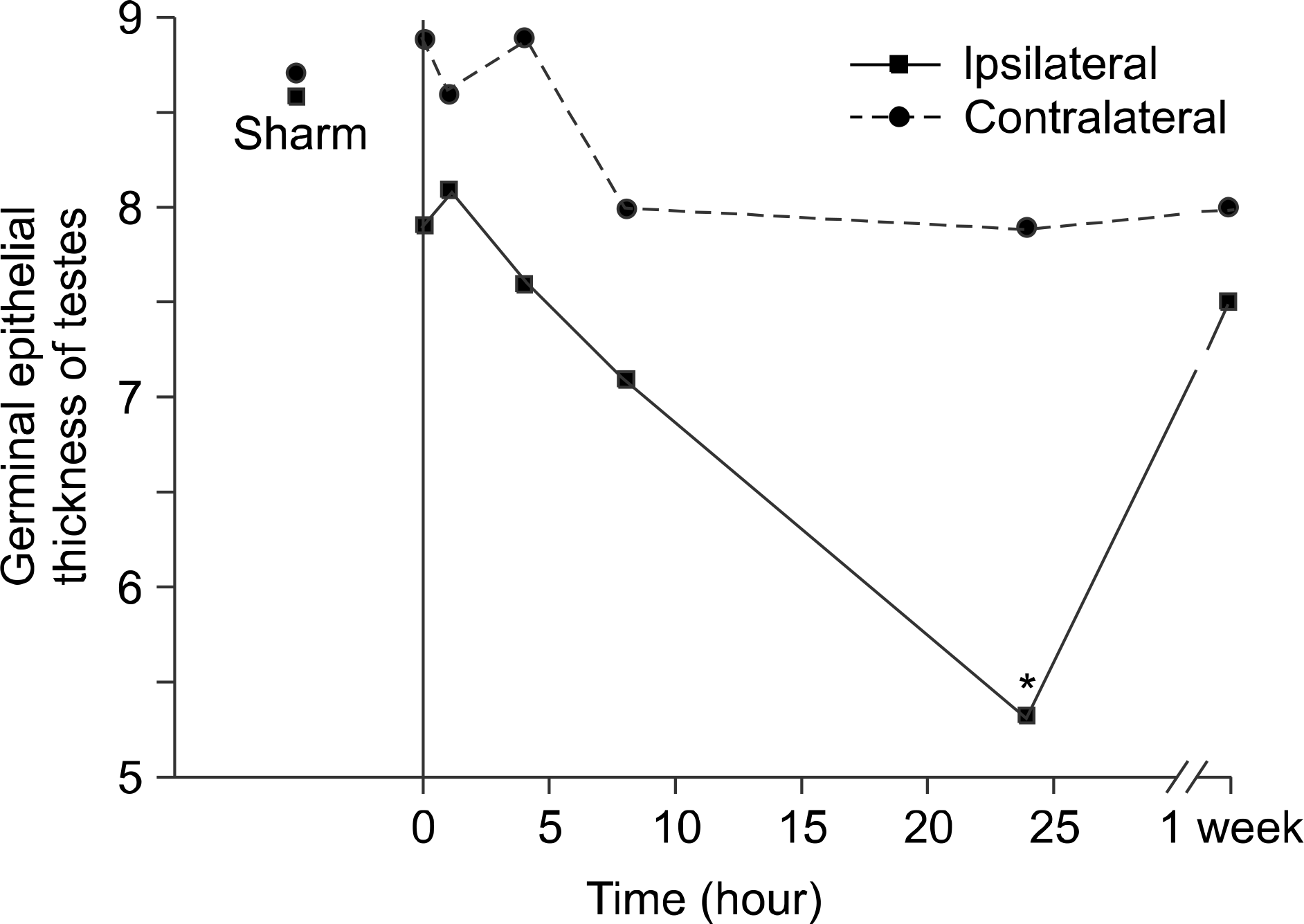 | Fig. 3.The germinal epithelial cell thickness of the ipsilateral testes taken 24 hours after repair of the torsion was significantly decreased. Asterisk indicated p<0.05, multiple comparison (Dunn procedure) with Kruskall-Wallis test. |
 | Fig. 4.Photomicrograph of unilateral and contralateral epididymides after testicular torsion and repair of the torsion. (A, B) Head of the ipsilateral and contralateral rat epididymides 0 hours after detorsion, demonstrating a normal ductal contour. (C) Head of the ipsilateral rat epididymis 4 hours after detorsion, demonstrating interstitial fibroblastic proliferation and hemorrhage. (D) Head of the contralateral rat epididymis 4 hours after detorsion, demonstrating a normal ductal contour compared with the ipsilateral epididymis. (E) Head of the ipsilateral rat epididymis 24 hours after detorsion, demonstrating more increased interstitial fibroblastic proliferation compared with the ipsilateral epididymis 8 hours after detorsion. (F) Head of the contralateral rat epididymis 24 hours after detorsion, demonstrating interstitial fibroblastic proliferation and hemorrhage, but less prominently than the ipsilateral epididymis after the same time. (G) Head of the ipsilateral rat epididymis 1 week after detorsion, demonstrating shortening of the tubular epithelial cell height and tubule dilatation. (H) Head of the contralateral rat epididymis 1 week after detorsion, demonstrating similar histologic findings shown in the ipsilateral epididymis at the same time (H&E, x200). |
Table 1.
The mean number of spermatids per tubule and histologic findings of ipsilateral and contralateral testes after testicular torsion and repair of the torsion
Table 2.
The mean seminiferous tubular diameter and germinal epithelial thickness of the ipsilateral and contralateral testes after testicular torsion and repair of the torsion




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download