Abstract
Purpose
We evaluated the differences in treatment outcomes between patients who complained of nocturia and patients who did not complain of nocturia after treatment with medication for benign prostatic hyperplasia (BPH) for >6 months. We also investigated the effectiveness of desmopressin on persistent nocturia after BPH medication.
Materials and Methods
One hundred forty-nine patients with 3 or more episodes of nocturia, despite treatment with BPH medications, were enrolled. Patients were divided into two groups according to complaints or absence of complaints of persistent nocturia. We compared differences of the International Prostate Symptom Score (IPSS), quality of life (QoL), and nocturia between the two groups. Patients who complained of persistent nocturia were subdivided into the following three groups after administration of desmopressin (0.2mg/day) with cessation of BPH medication: group I, decreased nocturia (>2 episodes per night) with desmopressin only; group II, decreased nocturia with desmopressin, but the BPH symptoms are aggravated, and the previous medication was added; group III, no change of nocturia despite desmopressin. We analyzed the differences in each group.
Results
Patients who complained of nocturia after BPH medication had a greater decrease in IPSS than those who did not complain of nocturia (p=0.047). Twenty percent (n=9) of the patients had decreased nocturia and were satisfied with desmopressin treatment, Twenty-four of the patients (53.3%) had decreased nocturia, but needed a combination with the previous BPH medication, while desmopressin was not effective in 26.7% (n=12) of the patients. The improvement of BPH after primary treatment was more evident in patients who experienced efficacy with desmopressin.
Conclusions
Desmopressin can be an effective treatment for persistent nocturia in patients with nocturnal polyuria components. The more improvement in BPH after primary treatment, the better the effects of desmopressin can be expected. We cannot overemphasize the importance of a voiding diary and analysis of nocturia.
Go to : 
References
1. Kim ET, Lee SI, Lee KS. The etiology and classification of nocturia in adults. Korean J Urol. 2001; 42:1075–9.
2. Johnson TM, Jones K, Williford WO, Kutner MH, Issa MM, Lepor H. Changes in nocturia from medical treatment of benign prostatic hyperplasia: secondary analysis of the department of Veterans Affairs Cooperative Study Trial. J Urol. 2003; 170:145–8.

3. Häkkinen JT, Hakama M, Shiri R, Auvinen A, Tammela TL, Koskimäki J. Incidence of nocturia in 50 to 80-year-old finnish men. J Urol. 2006; 176:2541–5.

4. Yu HJ, Chen FY, Huang PC, Chen HH, Chie WC, Liu CY. Impact of nocturia on symptom-specific quality of life among community-dwelling adults aged 40 years and older. Urology. 2006; 67:713–8.

5. Chang SC, Lin AT, Chen KK, Chang LS. Multifactorial nature of male nocturia. Urology. 2006; 67:541–4.

7. Britton JP, Dowell AC, Whelan P. Prevalence of urinary symptoms in men aged over 60. Br J Urol. 1990; 66:175–6.

8. Saito M, Kondo A, Kato T, Yamada Y. Frequency-volume charts: comparison of frequency between elderly and adult patients. Br J Urol. 1993; 72:38–41.

9. Johnson TM, Burrows PK, Kusek JW, Nyberg LM, Tenover JL, Lepor H, et al. The effect of doxazosin, finasteride and combination therapy on nocturia in men with benign prostatic hyperplasia. J Urol. 2007; 178:2045–51.

10. Bruskewitz RC, Larsen EH, Madsen PO, Dorflinger T. 3-year follow up of urinary symptoms after transurethral resection of the prostate. J Urol. 1986; 136:613–5.
11. Akbal C, Ekici S, Erkan I, Tekgul S. Intermittent oral desmopressin therapy for monosymptomatic primary nocturnal enuresis. J Urol. 2004; 171:2603–6.

12. Lee DH, Kim JC, Suh HJ, Kim HW, Hwang TK. The effect of desmopressin in nocturia. Korean J Urol. 1999; 40:613–6.
13. Nam SG, Moon DG, Kim JJ. Efficacy of desmopressin in treatment of adult nocturia. Korean J Urol. 2004; 45:49–55.
Go to : 
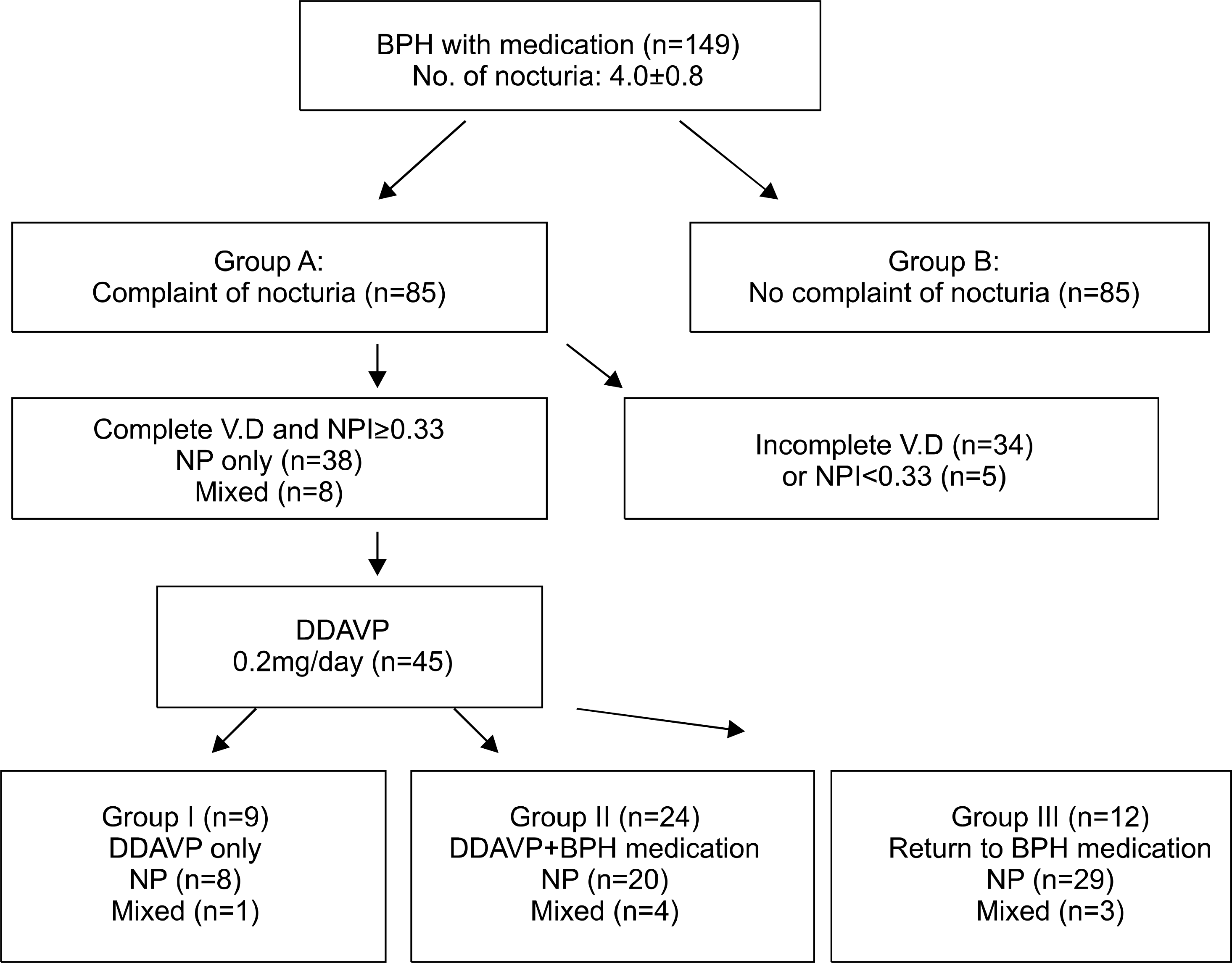 | Fig. 1.Progress of treatment for patients with persistent nocturia. BPH: benign prostatic hyperplasia, V.D: voiding diary, NPI: nocturnal polyuria index, NP: nocturnal polyuria, DDAVP: desmopressin acetate. |
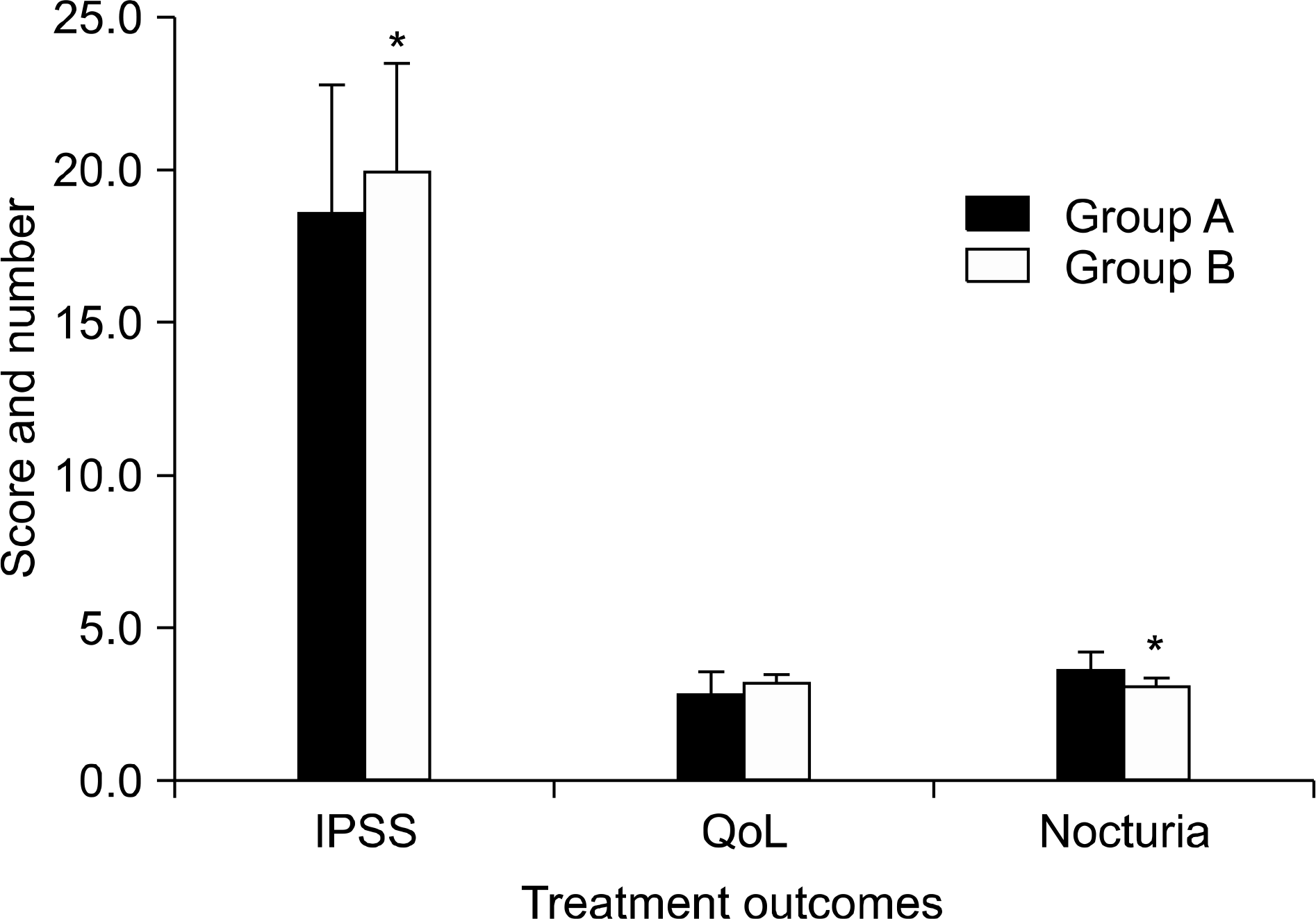 | Fig. 2.Comparison of the changes in IPSS, QoL, and number of nocturia after primary BPH treatment between groups A and B. ∗: p<0.05, IPSS: International Prostate Symptom Score, QoL: quality of life, BPH: benign prostatic hyperplasia. |
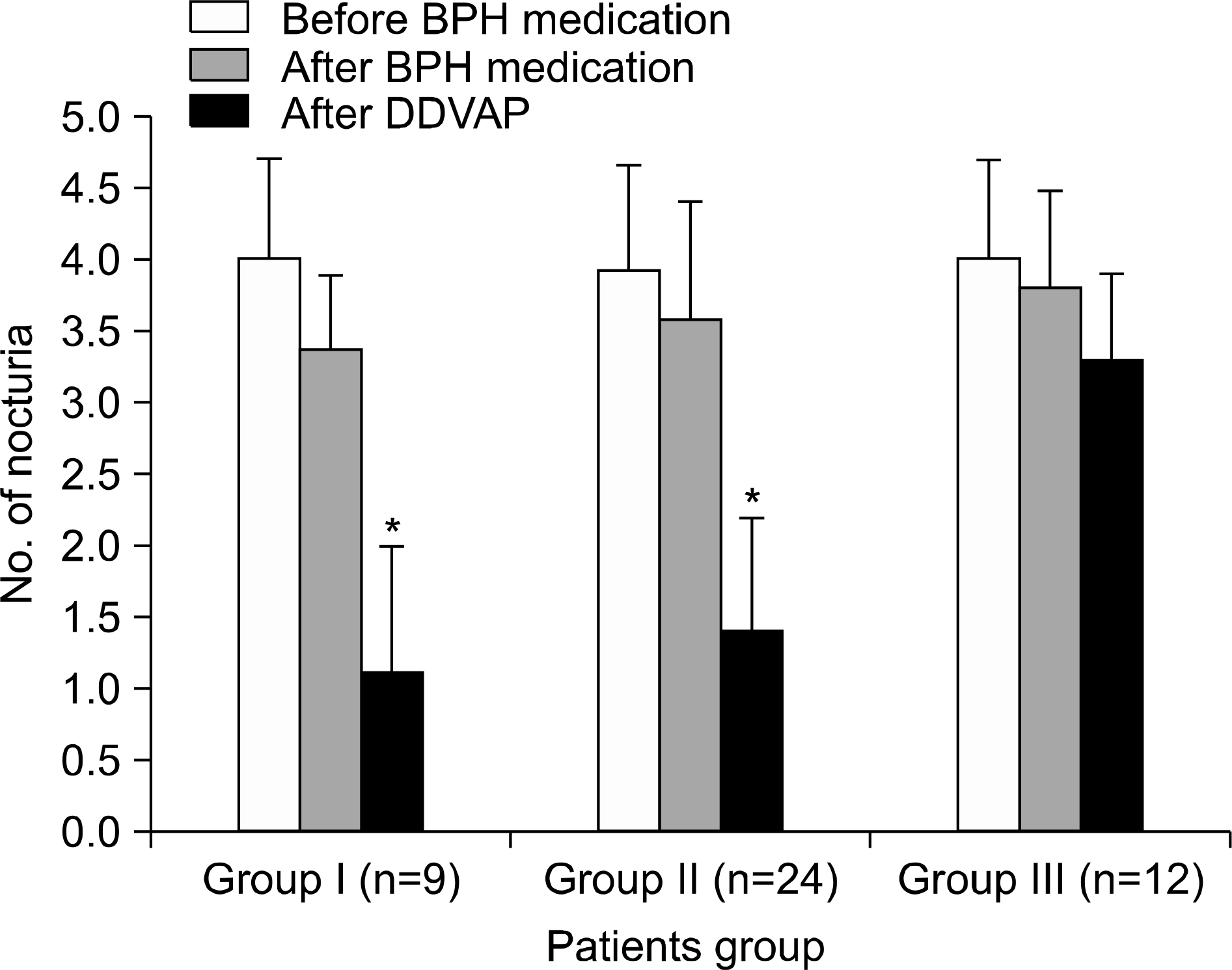 | Fig. 3.Treatment-related changes of nocturia. ∗: p<0.05, BPH: benign prostatic hyperplasia, DDAVP: desmopressin acetate. |
Table 1.
Comparison of primary BPH treatments in each group
Table 2.
The characteristics of groups A and B before primary BPH treatment
Table 3.
Comparison of the treatment outcomes before and after primary BPH treatment in groups A and B
Table 4.
The characteristics of subgroups I, II, and III before primary BPH treatment
Table 5.
Comparison of the treatment outcomes before and after primary BPH treatment in subgroups I, II, and III
Table 6.
Comparison of change-value before and after primary BPH treatment between the two groups (group I+II, DDAVP is effective; group III, DDAVP is not effective on nocturia)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download