Abstract
Purpose
Dihydrotestosterone (DHT) is key to the initiation and maintenance of abnormal prostatic growth in benign prostatic hyperplasia (BPH). Five α-reductase inhibitor reduces prostatic growth and serum prostate-specific antigen (PSA) by blocking the conversion of testosterone to DHT. Dutasteride is a dual (type 1 and 2) 5α-reductase inhibitor. We evaluated the effects of dutasteride on prostate volume, PSA, and PSA density in men with BPH.
Materials and Methods
A total of 83 men with a clinical diagnosis of BPH were treated with dutasteride and an alpha-blocker. We investigated the change in prostate volume, PSA, and PSA density 6 and 12 months after initiation of dutasteride therapy.
Results
After 6 months of dutasteride therapy, the total prostate volume was reduced from baseline by a mean of 15.46%, the PSA was reduced by a mean of 48.24%, and the PSA density was reduced by a mean of 37.97% (p<0.001). After 12 months of dutasteride therapy, the total prostate volume was reduced from baseline by a mean of 23.3%, the PSA was reduced by a mean of 52.57%, and the PSA density was reduced by a mean of 36.2% (p<0.001). There were no differences in the regression rate of PSA and PSA density, in contrast to prostate volume, between 6 and 12 months of dutasteride therapy by repeated measures ANOVA.
References
1. AUA Practice Guidelines Committee. AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: diagnosis and treatment recommendations. J Urol. 2003; 170:530–47.
2. Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001; 37(Suppl 8):S4–66.

3. Lieberman R. Evidence-based medical perspectives: the evolving role of PSA for early detection, monitoring of treatment response, and as a surrogate end point of efficacy for interventions in men with different clinical risk states for the prevention and progression of prostate cancer. Am J Ther. 2004; 11:501–6.

4. Bruchovsky N, Rennie PS, Batzold FH, Goldenberg SL, Fletcher T, McLoughlin MG. Kinetic parameters of 5 alpha-reductase activity in stroma and epithelium of normal, hyperplastic, and carcinomatous human prostates. J Clin Endocrinol Metab. 1988; 67:806–16.
5. Guess HA, Gormley GJ, Stoner E, Oesterling JE. The effect of finasteride on prostate specific antigen: review of available data. J Urol. 1996; 155:3–9.

6. Cho SH, Lee SK. The experience with combination of finasteride and tamsulosin on benign prostatic hyperplasia. Korean J Urol. 2003; 44:1110–5.
7. Soh BH, Lee JS, Chung BH. The changing pattern of serum prostate specific antigen in patients with benign prostatic hyperplasia after combined treatment with finasteride andα-blockers: the 3 year follow-up data. Korean J Urol. 2006; 47:372–6.
8. Marihart S, Harik M, Djavan B. Dutasteride: a review of current data on a novel dual inhibitor of 5alpha reductase. Rev Urol. 2005; 7:203–10.
9. Sawaya ME, Price VH. Different levels of Salpha-reductase type I and II, aromatase, and androgen receptor in hair follicles of women and men with androgenetic alopecia. J Invest Dermatol. 1997; 109:296–300.
10. McConnell JD, Roehrborn CG, Bautista OM, Andriole GL Jr, Dixon CM, Kusek JW, et al. The longterm effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003; 349:2387–98.

11. Debruyne F, Barkin J, van Erps P, Reis M, Tammela TL, Roehrborn C, et al. Efficacy and safety of longterm treatment with the dual 5 alpha-reductase inhibitor dutasteride in men with symptomatic benign prostatic hyperplasia. Eur Urol. 2004; 46:488–94.
12. McConnell JD, Bruskewitz R, Walsh P, Andriole G, Lieber M, Holtgrewe HL, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med. 1998; 338:557–63.
13. Basillote JB, Armenakas NA, Hochberg DA, Fracchia JA. Influence of prostate volume in the detection of prostate cancer. Urology. 2003; 61:167–71.

14. Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003; 349:215–24.

15. Andriole GL, Roehrborn C, Schulman C, Slawin KM, Somerville M, Rittmaster RS. Effect of dutasteride on the detection of prostate cancer in men with benign prostatic hyperplasia. Urology. 2004; 64:537–41.

16. Andriole GL, Guess HA, Epstein JI, Wise H, Kadmon D, Crawford ED, et al. Treatment with finasteride preserves usefulness of prostate-specific antigen in the detection of prostate cancer: results of a randomized, double-blind, placebocontrolled clinical trial. PLESS Study Group. Proscar Longterm Efficacy and Safety Study. Urology. 1998; 52:195–201.
17. Andriole GL, Marberger M, Roehrborn CG. Clinical usefulness of serum prostate specific antigen for the detection of prostate cancer is preserved in men receiving the dual 5alpha-reductase inhibitor dutasteride. J Urol. 2006; 175:1657–62.
18. Bazinet M, Meshref AW, Trudel C, Aronson S, Peloquin F, Nachabe M, et al. Prospective evaluation of prostate-specific antigen density and systematic biopsies for early detection of prostate carcinoma. Urology. 1994; 43:44–51.
19. Yoon SW, Kim JI, Park SS. Change of PSA density after finasteride therapy in patients with benign prostatic hyperplasia. Korean J Urol. 2002; 43:19–22.
Fig. 1.
Mean percent change from baseline in (A) prostate volume, (B) PSA, and (C) PSA density after dutasteride treatment during 12 months. PSA: prostate-specific antigen.
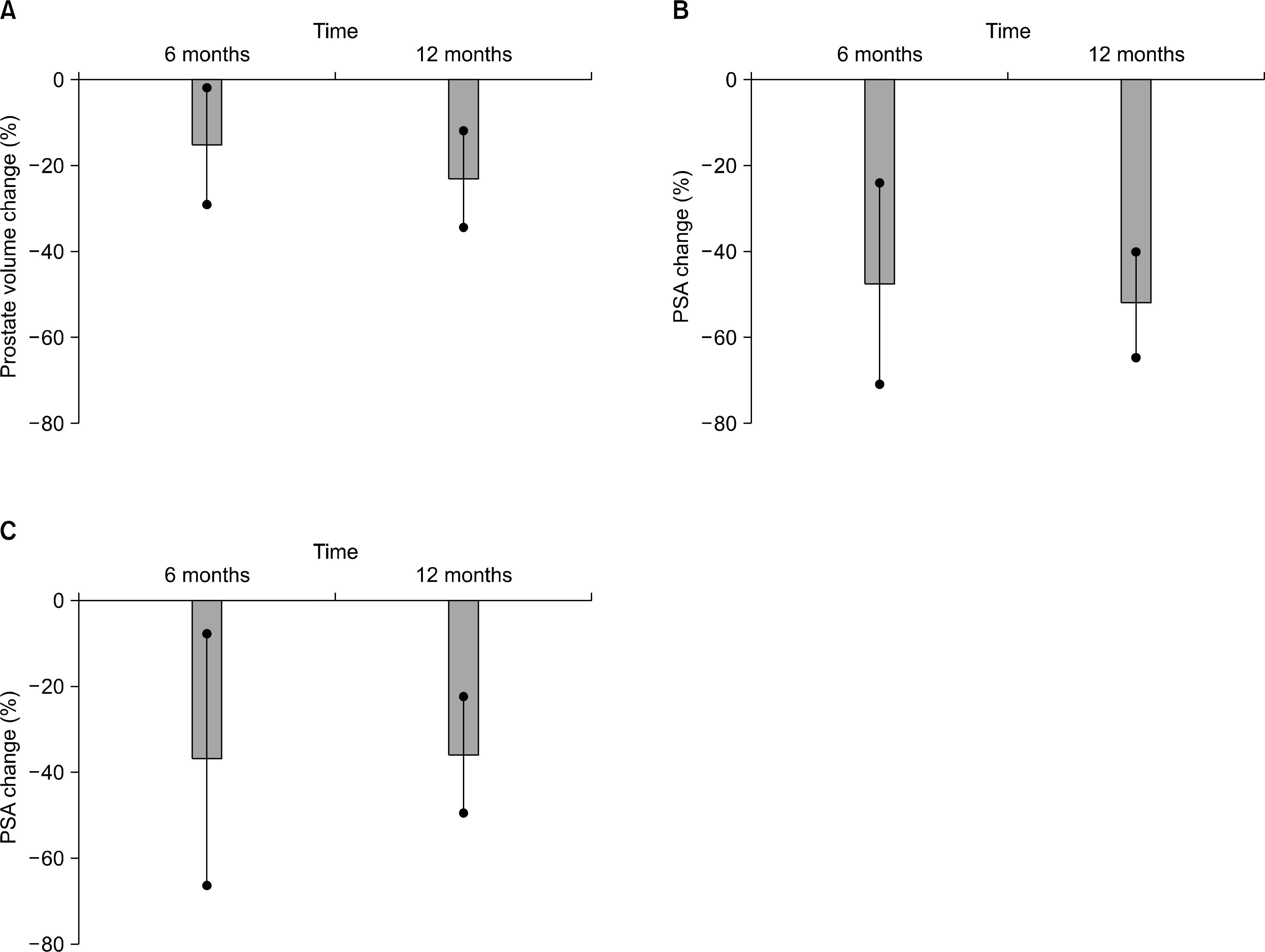
Fig. 2.
(A) Prostate volume, (B) PSA, and (C) PSA density pattern according to the follow-up period after dutasteride treatment. PSA: †: 6 vs. 12 prostate-specific antigen. ∗: baseline vs. 6 months, months.
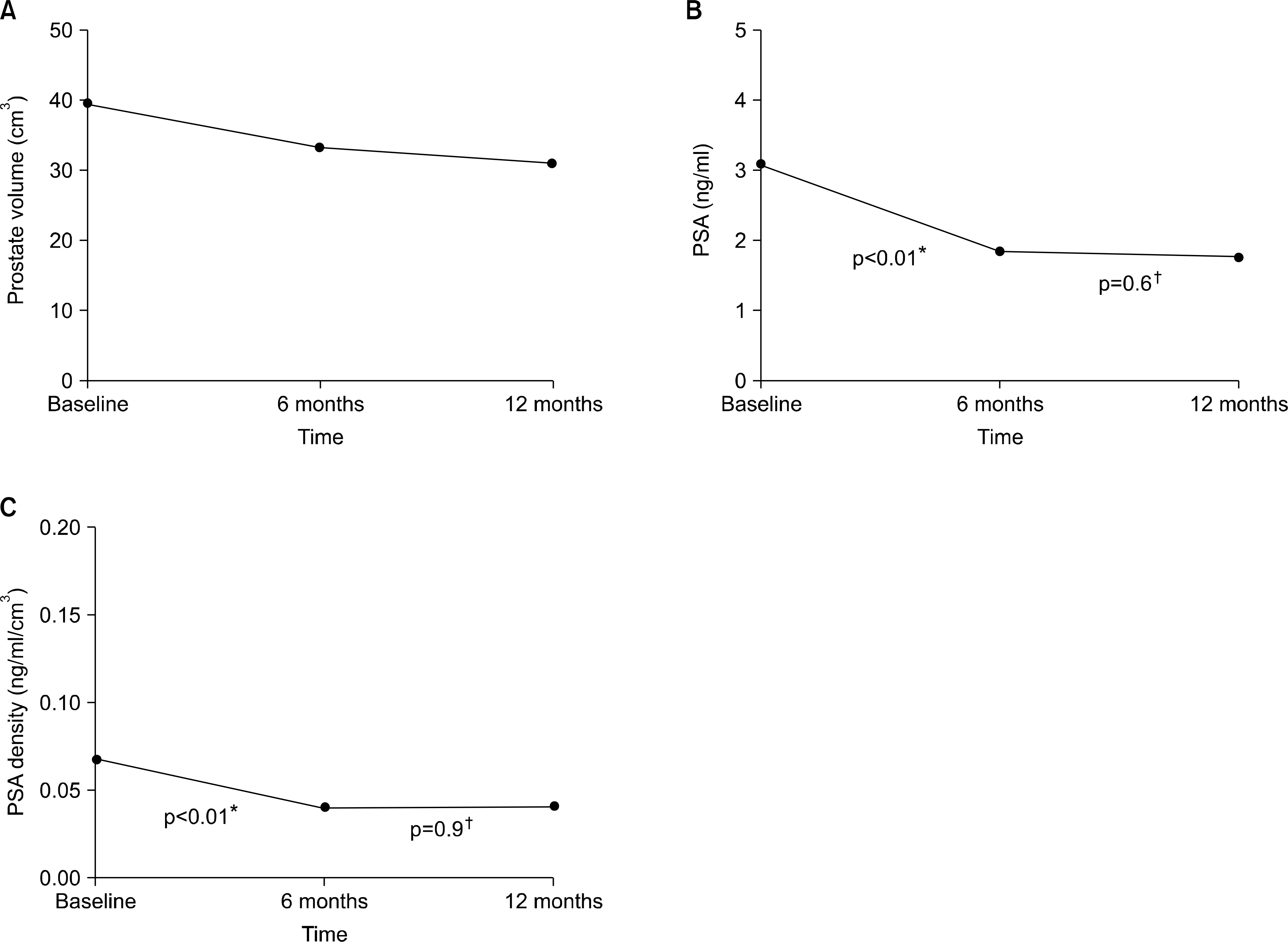
Table 1.
Summary of baseline characteristics of patients
Table 2.
Regression rate (%) in each group after dutasteride treatment




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download