Abstract
Purpose
The HedgehogGli (HHGli) signaling pathway controls many aspects of tissue patterning, cell proliferation, differentiation and regeneration, and regulates the number of cells in various organs. Inappropriate and uncontrolled activation of the HHGli signaling pathway has been demonstrated in a variety of human cancers. The Gli1, Gli2, and Gli3 genes encoding the Gli family transcription factors play a role as HH effectors. This study examined the significance in Gli2 and Gli3 expression in human bladder cancer.
Materials and Methods
The tumor tissues were obtained from 144 patients with a primary bladder cancer. The mRNA levels of Gli2, and Gli3 were examined using a real-time polymerase chain reaction (PCR) assay in 144 tumor specimens, and immunohistochemical staining was performed on 127 tumor paraffin blocks. The relationships between their expression and the pathological or clinical characteristics, such as tumor stage, grade, recurrence and progression were also analyzed.
Results
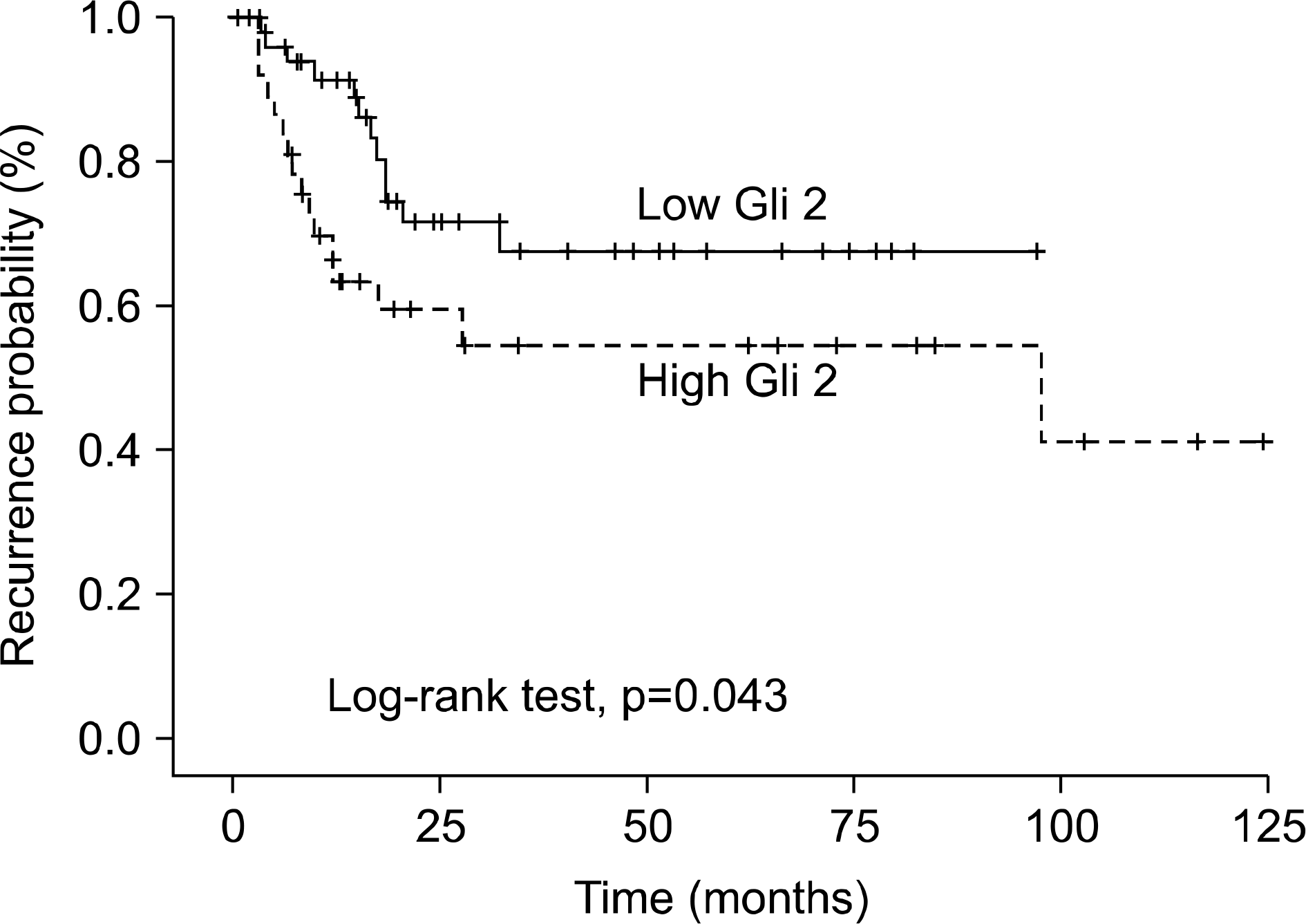
Gli2 mRNA expression was higher in the invasive bladder tumors than in the superficial bladder tumors (p<0.001) but, there was no difference in Gli3 mRNA expression according to the tumor stage and grade. The multivariate Cox regression model revealed that Gli2 mRNA expression (hazards ratio (HR): 2.329, 95% confidence interval (CI): 1.043-5.202, p=0.039) was the only strong predictor of superficial bladder tumor recurrence. Kaplan-Meier analysis also showed identical results (log-rank test, p=0.043).
REFERENCES
1.Kim WJ., Chung JI., Hong JH., Kim CS., Jung SI., Yoon DK. Epidemiological study for urologic cancer in Korea (1998-2002). Korean J Urol. 2004. 45:1081–8.
2.Hudson MA., Catalona WJ. Urothelial tumors of the bladder, upper tracts and prostate. Gillenwater JY, Grayhack JT, editors. editors.Adult and pediatric urology. 3rd ed.St. Louis: Mosby;1996. 1379-464.
3.Messing EM. Urothelial tumors of the bladder. Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. editors.Campbell-Walsh urology. 9th ed.Pennsylvania: Saunders;2006. 2426-7.
4.Beachy PA., Karhadkar SS., Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004. 432:324–31.

6.Ingham PW., McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001. 15:3059–87.

7.Berman DM., Karhadkar SS., Hallahan AR., Pritchard JI., Eberhart CG., Watkins DN, et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002. 297:1559–61.

8.Thayer SP., di Magliano MP., Heiser PW., Nielsen CM., Roberts DJ., Lauwers GY, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003. 425:851–6.

9.Ruiz i Altaba A., Palma V., Dahmane N. Hedgehog-Gli signalling and the growth of the brain. Nat Rev Neurosci. 2002. 3:24–33.
11.Sinicrope FA., Ruan SB., Cleary KR., Stephens LC., Lee JJ., Levin B. Bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995. 55:237–41.
12.Bland JM., Altman DG. Transformations, means, and confidence intervals. BMJ. 1996. 312:1079.
13.Sylvester RJ., van der Meijden AP., Oosterlinck W., Witjes JA., Bouffioux C., Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006. 49:466–75.

14.Marigo V., Davey RA., Zuo Y., Cunningham JM., Tabin CJ. Biochemical evidence that patched is the Hedgehog receptor. Nature. 1996. 384:176–9.

15.Bhardwaj G., Murdoch B., Wu D., Baker DP., Williams KP., Chadwick K, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001. 2:172–80.

16.Lai K., Kaspar BK., Gage FH., Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003. 6:21–7.

17.Machold R., Hayashi S., Rutlin M., Muzumdar MD., Nery S., Corbin JG, et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003. 39:937–50.

18.Hooper JE., Scott MP. The Drosophila patched gene encodes a putative membrane protein required for segmental patterning. Cell. 1989. 59:751–65.

19.Ha YS., Yun SJ., Kim YJ., Lee SC., Kim WJ. Utility of Smo as a prognostic marker for human bladder tumors. Korean J Urol. 2007. 48:997–1003.

20.Katoh Y., Katoh M. Hedgehog signaling pathway and gastrointestinal stem cell signaling network (review). Int J Mol Med. 2006. 18:1019–23.

21.Xie J., Murone M., Luoh SM., Ryan A., Gu Q., Zhang C, et al. Activating smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998. 391:90–2.

22.Sasaki H., Nishizaki Y., Hui C., Nakafuku M., Kondoh H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development. 1999. 126:3915–24.

23.Shin SH., Kogerman P., Lindstrom E., Toftgard R., Biesecker LG. GLI3 mutations in human disorders mimic Drosophila cubitus interruptus protein functions and localization. Proc Natl Acad Sci USA. 1999. 96:2880–4.

24.Stecca B., Ruiz i Altaba A. The therapeutic potential of modulators of the Hedgehog-Gli signaling pathway. J Biol. 2002. 1:9.
25.Kinzler KW., Bigner SH., Bigner DD., Trent JM., Law ML., O'Brien SJ, et al. Identification of an amplified, highly expressed gene in a human glioma. Science. 1987. 236:70–3.

26.Snijders AM., Schmidt BL., Fridlyand J., Dekker N., Pinkel D., Jordan RC, et al. Rare amplicons implicate frequent deregulation of cell fate specification pathways in oral squamous cell carcinoma. Oncogene. 2005. 24:4232–42.

Fig. 2.
Immunohistochemical staining of Gli2 in the normal bladder mucosa (A), superficial bladder tumor (B), invasive bladder tumor (C) and Gli3 in normal bladder mucosa (D), superficial bladder tumor (E), invasive bladder tumor (F).

Table 1.
Clinical and pathological features of the primary bladde transitional cell carcinomas
Table 2.
Gli2 and Gli3 mRNA expression levels of primary bladder transitional cell carcinomas
| Variables | Gli2* | p-value† | Gli3* | p-value† |
|---|---|---|---|---|
| Stage | <0.001 | 0.649 | ||
| Superficial | 3.8 (2.9-4.9) | 6.3 (4.9-8.2) | ||
| Invasive | 13.9 (9.3-20.7) | 7.0 (4.6-10.8) | ||
| Grade | 0.155 | 0.617 | ||
| Low | 5.3 (3.9-7.1) | 6.9 (5.3-8.9) | ||
| High | 7.6 (5.0-11.5) | 6.1 (4.0-9.3) |
Table 3.
Multivariate analysis for the prediction the recurrence of superficial bladder cancer
Table 4.
Gli2 and Gli3 immunohistochemical staining scores in primary bladder transitional cell carcinomas
| Variables | No. (%) | Gli2 | p-value* | Gli3 | p-value* |
|---|---|---|---|---|---|
| Stage | 0.008 | 0.039 | |||
| Superficial | 76 (59.8) | 5.47±2.16 | 6.03±2.15 | ||
| Invasive | 51 (40.2) | 6.21±2.27 | 6.97±2.67 | ||
| Grade | 0.023 | 0.010 | |||
| Low | 80 (63.0) | 5.34±1.77 | 5.96±2.37 | ||
| High | 47 (37.6) | 6.28±2.62 | 7.11±2.51 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download