Abstract
Purpose
This study examined the expression of clusterin and Ki-67 in human transitional cell carcinoma (TCC). In addition, the relationship of clusterin and Ki-67 expression with the clinicopathological factors and prognosis of human TCC was investigated.
Materials and Methods
149 human TCC tissues were obtained from 149 patients who underwent a radical cystectomy (n=81) or transurethral resection (n=68). The expression of clusterin and Ki-67 was analyzed using immunohistochemical staining. The results were evaluated with respect to the clinicopathological factors.
Results
Positive clusterin expression was observed in 21.1% of the total TCC tissues. The expression of clusterin was not significantly related to age, gender, tumor stage and grade. However, recurrence-free survival rate of the patients with positive clusterin expression was significantly lower than that of patients with negative clusterin expression (p=0.02). The expression level of Ki-67 in the TCC tissues was associated with the tumor stage (p<0.001) and grade (p<0.001), but not with age and gender. Furthermore, the recurrence-free survival rate of patients with strong Ki-67 expression was significantly lower than that of patients with weak Ki-67 expression (p<0.001). The expression of clusterin was not significantly related to the level of Ki-67 expression. However, in the patients showing strong Ki-67 expression, the recurrence-free survival rate of the patients with positive clusterin expression was significantly lower than that of the patients with negative clusterin expression (p<0.001).
REFERENCES
1.Joung JY., Seo HK., Chung JS., Hwang HS., Hong EK., Lee KH. Clinical efficacy of Ki-67, apoptotic index, bcl-2, and p53 in bladder tumor: correlation with stage, histologic grade and recurrence rate. Korean J Urol Oncol. 2003. 1:376–81.
2.Weinberg RA. Oncogenes, antioncogenes, and the molecular bases of multistep carcinogenesis. Cancer Res. 1989. 49:3713–21.
3.Sylvester SR., Morales C., Oko R., Griswold MD. Localization of sulfated glycoprotein-2 (clusterin) on spermatozoa and in the reproductive tract of the male rat. Biol Reprod. 1991. 45:195–207.
4.Fritz IB., Burdzy K., Setchell B., Blaschuk O. Ram rete testis fluid contains a protein (clusterin) which influences cell-cell interactions in vitro. Biol Reprod. 1983. 28:1173–88.
5.Parczyk K., Pilarsky C., Rachel U., Koch-Brandt C. Gp80 (clusterin; TRPM-2) mRNA level is enhanced in human renal clear cell carcinomas. J Cancer Res Clin Oncol. 1994. 120:186–8.

6.Sensibar JA., Sutkowski DM., Raffo A., Buttyan R., Griswold MD., Sylvester SR, et al. Prevention of cell death induced by tumor necrosis factor alpha in LNCaP cells by overexpression of sulfated glycoprotein-2 (clusterin). Cancer Res. 1995. 55:2431–7.
7.Leskov KS., Klokov DY., Li J., Kinsella TJ., Boothman DA. Synthesis and functional analyses of nuclear clusterin, a cell death protein. J Biol Chem. 2003. 278:11590–600.

8.Kruger S., Mahnken A., Kausch I., Feller AC. Value of clusterin immunoreactivity as a predictive factor in muscle-invasive urothelial bladder carcinoma. Urology. 2006. 67:105–9.

9.Miyake H., Gleave M., Kamidono S., Hara I. Overexpression of clusterin in transitional cell carcinoma of the bladder is related to disease progression and recurrence. Urology. 2002. 59:150–4.

10.Yoon JH., Lee JH., Yeom BW., Won NH., Yoon DK. Expression of osteopontin and clusterin in transitional cell carcinoma of the bladder: comparison to the pathologic stage. Korean J Urol. 2005. 46:341–6.
11.Park HJ., Lee HJ., Yum YH., Kang JY., Yoo TK. Clusterin expression and apoptosis in transitional cell carcinoma of the bladder. Korean J Urol. 2007. 48:402–7.

12.Mellon K., Neal DE., Robinson MC., Marsh C., Wright C. Cell cycling in bladder carcinoma determined by monoclonal antibody Ki67. Br J Urol. 1990. 66:281–5.

13.Lakins J., Bennett SA., Chen JH., Arnold JM., Morrissey C., Wong P, et al. Clusterin biogenesis in altered during apoptosis in the regressing rat ventral prostate. J Biol Chem. 1998. 273:27887–95.
14.Pucci S., Bonanno E., Pichiorri F., Angeloni C., Spagnoli LG. Modulation of different clusterin isoforms in human colon tumorigenesis. Oncogene. 2004. 23:2298–304.

15.July LV., Beraldi E., So A., Fazli L., Evans K., English JC, et al. Nucleotide-based therapies targeting clusterin chemosen-sitize human lung adenocarcinoma cells both in vitro and in vivo. Mol Cancer Ther. 2004. 3:223–32.
16.Zellweger T., Kiyama S., Chi K., Miyake H., Adomat H., Skov K, et al. Overexpression of the cytoprotective protein clusterin decreases radiosensitivity in the human LNCaP prostate tumour model. BJU Int. 2003. 92:463–9.

17.Scaltriti M., Brausi M., Amorosi A., Caporali A., D'Arca D., Astancolle S, et al. Clusterin (SGP-2, ApoJ) expression is downregulated in low- and high-grade human prostate cancer. Int J Cancer. 2004. 108:23–30.

18.Miyake H., Hara S., Zellweger T., Kamidono S., Gleave ME., Hara I. Acquisition of resistance to Fas-mediated apoptosis by overexpression of clusterin in human renal-cell carcinoma cells. Mol Urol. 2001. 5:105–11.

19.Steinberg J., Oyasu R., Lang S., Sintich S., Rademaker A., Lee C, et al. Intracellular levels of SGP-2 (clusterin) correlate with tumor grade in prostate cancer. Clin Cancer Res. 1997. 3:1707–11.
20.Kurahashi T., Muramaki M., Yamanaka K., Hara I., Miyake H. Expression of the secreted form of clusterin protein in renal cell carcinoma as a predictor of disease extension. BJU Int. 2005. 96:895–9.

21.Miyake H., Hara I., Kamidono S., Gleave ME. Synergistic chemsensitization and inhibition of tumor growth and meta-stasis by the antisense oligodeoxynucleotide targeting clusterin gene in a human bladder cancer model. Clin Cancer Res. 2001. 7:4245–52.
22.Gerdes J., Schwab U., Lemke H., Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983. 31:13–20.

23.Burger PC., Shibata T., Kleihues P. The use of the monoclonal antibody Ki-67 in the identification of proliferating cells: application to surgical neuropathology. Am J Surg Pathol. 1986. 10:611–7.

24.Cohen MB., Waldman FM., Carroll PR., Kerschmann R., Chew K., Mayall BH. Comparison of five histopathologic methods to assess cellular proliferation in transitional cell carcinoma of the urinary bladder. Hum Pathol. 1993. 24:772–8.

25.Okamura K., Miyake K., Koshikawa T., Asai J. Growth fractions of transitional cell carcinomas of the bladder defined by the monoclonal antibody Ki-67. J Urol. 1990. 144:875–8.

26.Oosterhuis JW., Schapers RF., Janssen-Heijnen ML., Smeets AW., Pauwels RP. MIB-1 as a proliferative marker in transitional cell carcinoma of the bladder: clinical significance and comparison with other prognostic factors. Cancer. 2000. 88:2598–605.
Fig. 3.
Recurrence-free survival of the patients with a transitional cell carcinoma according to the clusterin expression status (p=0.02).
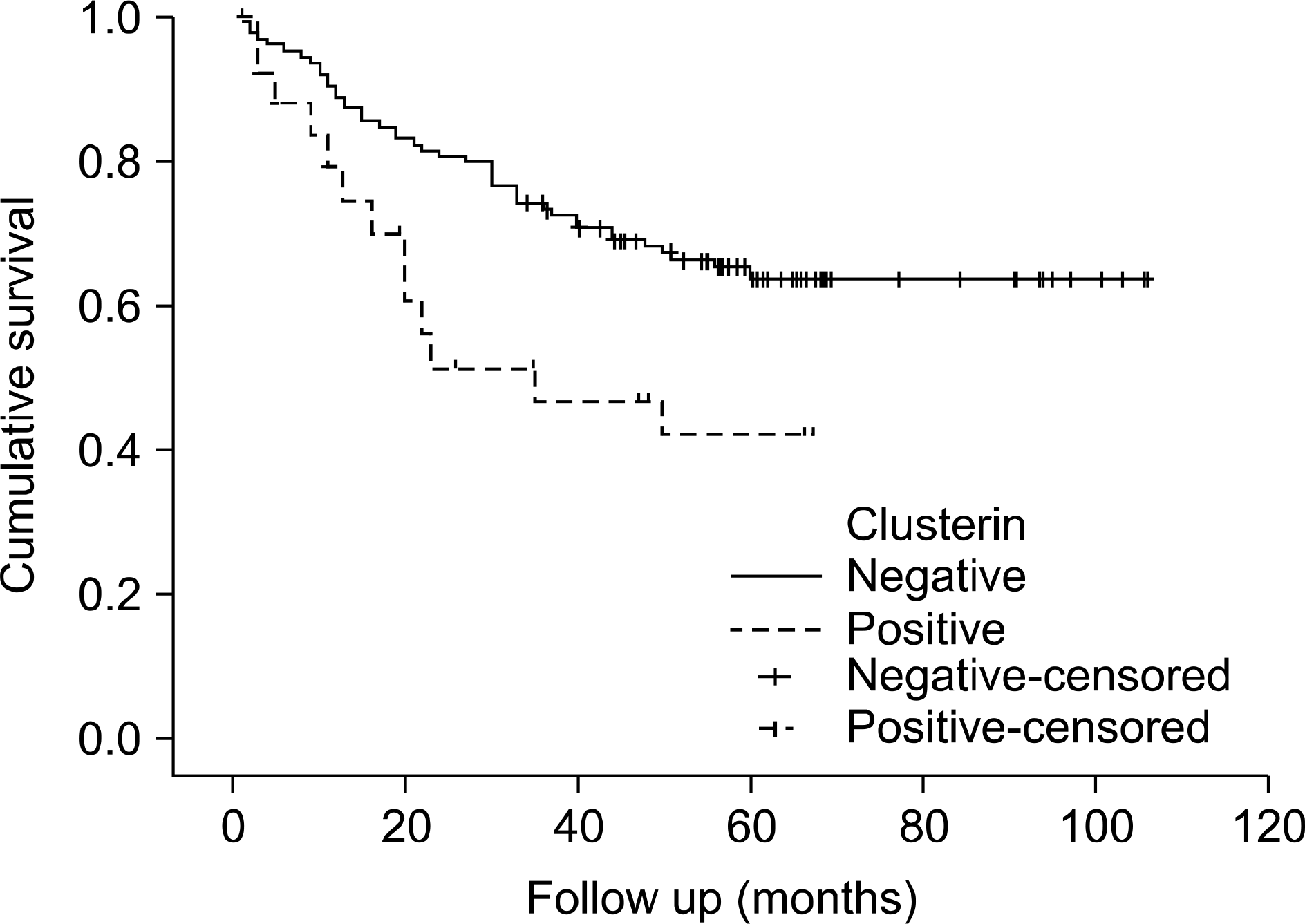
Fig. 4.
Recurrence-free survival of patients with transitional cell carcinoma according to the Ki-67 labeling index (p=0.003).
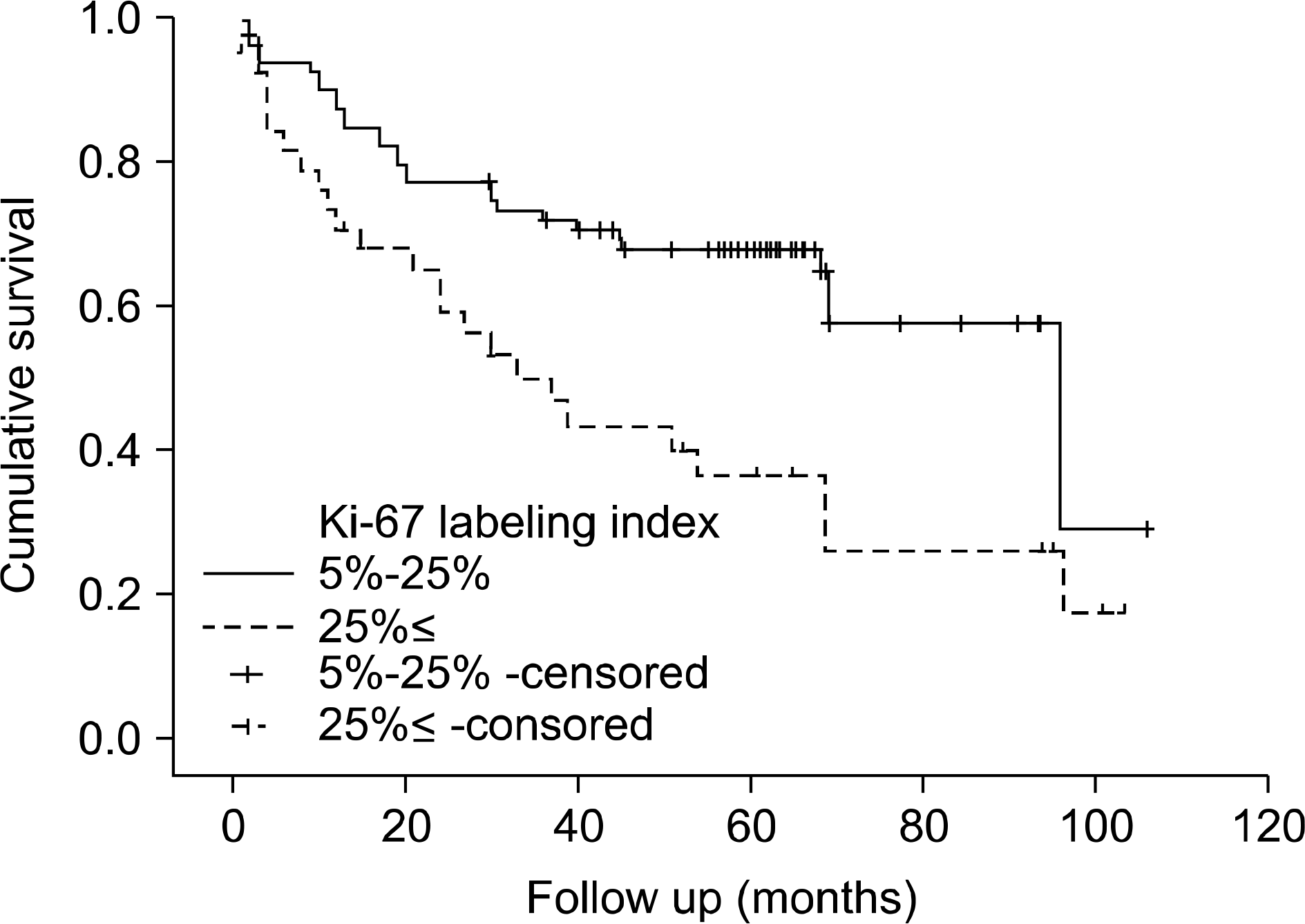
Fig. 5.
Recurrence-free survival of the patients with a transitional cell carcinoma according to the Ki-67 labeling index and clusterin expression (p=0.019).
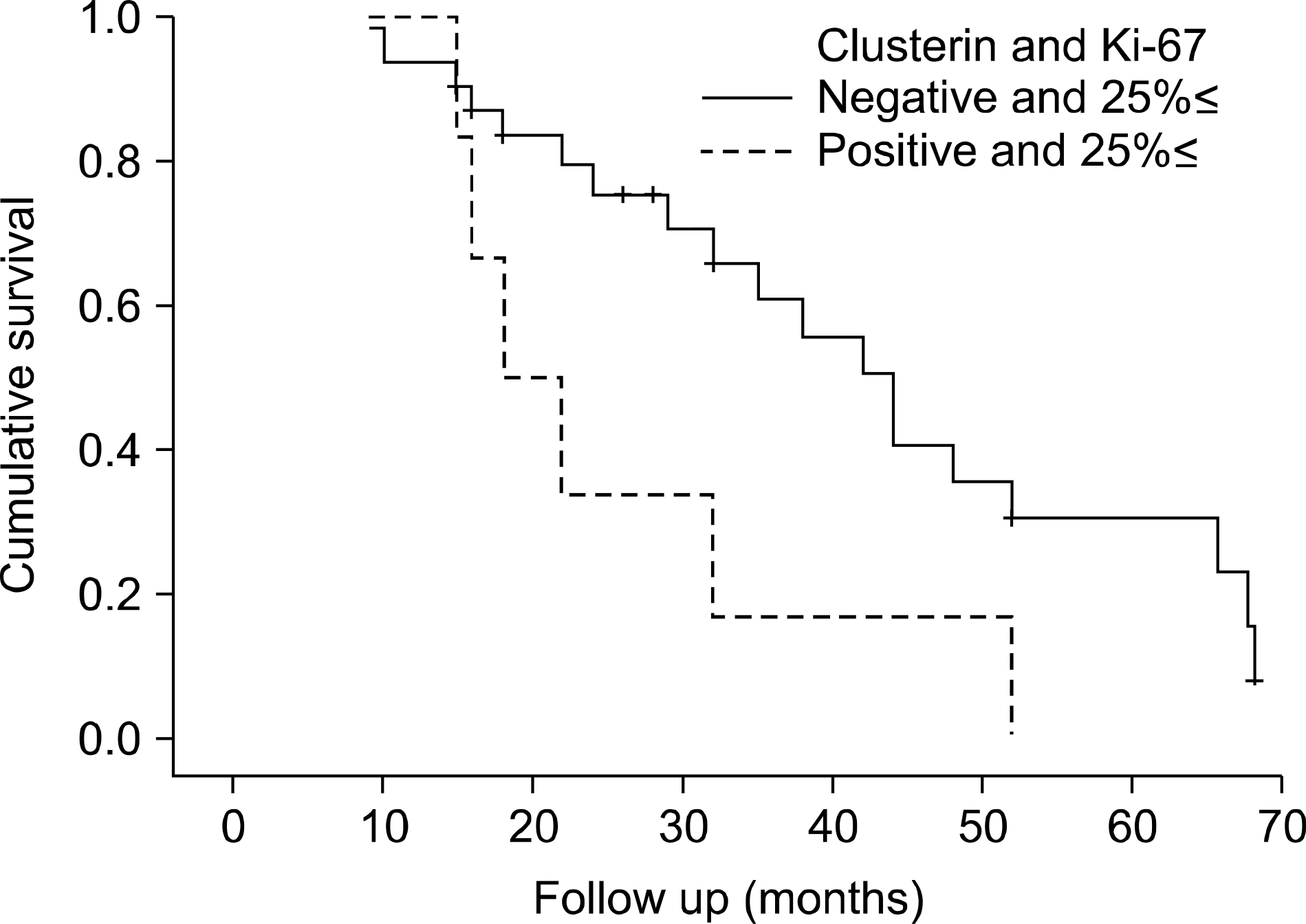
Table 1.
The correlation of clusterin expression in transitional cell carcinoma tissues, with the clinicopathological factors and cell proliferative activity
| Variables | n |
Clusterin staining extents, n (%) |
||
|---|---|---|---|---|
| Negative | Positive | p-value | ||
| Age (years) | 0.753* | |||
| ≤60 | 47 | 38 (80.9) | 9 (19.1) | |
| >60 | 102 | 85 (83.3) | 17 (16.7) | |
| Gender | 0.545* | |||
| Male | 99 | 81 (81.8) | 18 (18.2) | |
| Female | 50 | 42 (84.0) | 8 (16.0) | |
| Stage | 0.092† | |||
| pTa, pT1 | 74 | 61 (82.4) | 13 (17.6) | |
| pT2 | 43 | 32 (74.4) | 11 (25.6) | |
| pT3 | 32 | 30 (93.8) | 2 (6.2) | |
| WHO classification | 0.473† | |||
| Grade 1 | 23 | 21 (91.3) | 2 (8.7) | |
| Grade 2 | 76 | 62 (81.6) | 14 (18.4) | |
| Grade 3 | 50 | 40 (80.0) | 10 (20.0) | |
Table 2.
Results of clusterin immunohistochemical staining in the normal bladder and transitional cell carcinoma tissues
| Clusterin staining extents | Normal bladder |
TCC |
p-value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Negative (<5%) | 27 | 100.0 | 123 | 78.9 | |
| Positive (≥5%) | 0 | 0.0 | 26 | 21.1 | |
| Total | 27 | 100.0 | 149 | 100.0 | 0.016* |
Table 3.
Multivariate analysis of the recurrence-free survival according to various factors using the Cox’s proportional hazard regression model
Table 4.
The correlation of the Ki-67 labeling index in transitional cell carcinoma tissues with the clinicopathological factors and cell proliferative activity
| Variables | n |
Ki-67 labeling index, n (%) |
|||
|---|---|---|---|---|---|
| <5% | 5-25% | >25% | p-value | ||
| Age (years) | 0.066* | ||||
| ≤60 | 47 | 8 (17.0) | 31 (66.0) | 8 (17.0) | |
| ≥61 | 102 | 24 (23.5) | 47 (46.1) | 31 (30.4) | |
| Gender | 0.409* | ||||
| Male | 99 | 23 (23.2) | 48 (48.5) | 28 (28.3) | |
| Female | 50 | 9 (18.0) | 30 (60.0) | 11 (22.0) | |
| Stage | <0.001* | ||||
| pTa, pT1 | 74 | 23 (31.0) | 42 (56.8) | 9 (12.2) | |
| pT2 | 43 | 7 (16.2) | 26 (60.5) | 10 (23.3) | |
| pT3 | 32 | 2 (6.2) | 10 (31.3) | 20 (62.5) | |
| WHO | <0.001* | ||||
| classification | |||||
| Grade 1 | 23 | 12 (52.2) | 10 (43.5) | 1 (4.3) | |
| Grade 2 | 76 | 11 (14.4) | 48 (63.2) | 17 (22.4) | |
| Grade 3 | 50 | 9 (18.0) | 20 (40.0) | 21 (42.0) | |
Table 5.
Ki-67 labeling index in the normal bladder and transitional cell carcinoma tissues
| Ki-67 labeling index |
Normal bladder |
TCC |
p-value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| <5% | 24 | 88.9 | 32 | 21.5 | |
| 5-25% | 3 | 11.1 | 78 | 52.3 | |
| >25% | 0 | 0.0 | 39 | 26.2 | |
| Total | 27 | 100.0 | 149 | 100.0 | <0.001* |
Table 6.
The correlation of the Ki-67 labeling index with clusterin expression in transitional cell carcinoma tissues
| Variables | n |
Ki-67 labeling index, n (%) |
|||
|---|---|---|---|---|---|
| <5% | 5-25% | >25% | p-value | ||
| Clusterin | 0.92* | ||||
| staining extents | |||||
| Negative | 123 | 26 (21.1) | 64 (52.1) | 33 (26.8) | |
| Positive | 26 | 6 (23.1) | 14 (53.8) | 6 (23.1) | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download