Abstract
Purpose
The increased use of antibiotics may be the main factor responsible for the development and spread of bacterial resistance. This study demonstrated the relation between quinolone use and the rate of isolating ciprofloxacin-resistant (CIPRO-R) Escherichia coli (E.coli) in patients with urinary tract infection (UTI).
Materials and Methods
From 2001 to 2006, we determined antimicrobial use for 2,803 in terms of the defined daily dose (DDD) and the antimicrobial use density (AUD), and we surveyed the isolation rates of CIPRO-R E.coli in UTIs in both inpatients and outpatients. We also analyzed the correlation between the number of prescriptions and the resistance rates.
Results
Of the 637 (22.7%) CIPRO-R E.coli isolates, 297 (46.6%) were from inpatients and 340 (53.4%) were from outpatients. There was a statistically significant correlation between the rate of isolating CIPRO-R E.coli and the amount of quinolone use for the inpatients (r=0.815, p<0.05) as well as the outpatients (r=0.804, p<0.05). A logistic regression analysis identified previous quinolone use as the independent risk factor (odd ratio: 2.604 [95% confidence interval (CI): 1.639–4.137]) for CIPRO-R E.coli in inpatients. Also, these CIPRO-R E.coli showed low sensitivity to ampicillin and tri-methoprim/sufamethoxazole (TMP/SMX) in the inpatients (10.4%, 27.3%) and outpatients (5.1%, 27.1%), respectively.
Conclusions
Our study shows a significant correlation between ciprofloxacin resistance and quinolone use, and previous quinolone use seems to be the risk factor for CIPRO-R E.coli bacteriuria. It is necessary to keep antimictrobial therapy under constant surveillance for the prevention of CIPRO-R E.coli.
Go to : 
References
1. Cohen ML. Antimicrobial resistance: prognosis for public health. Trends Microbiol. 1994; 2:422–5.

2. Warren JW, Abrutyn E, Hebel JR, Johnson JR, Schaeffer AJ, Stamm WE. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA). Clin Infect Dis. 1999; 29:745–58.
3. Lee SJ, Cho YH, Kim BW, Lee JG, Jung SI, Lee SD, et al. A multicenter study of antimicrobial susceptibility of uropathogens causing acute uncomplicated cystitis in women. Korean J Urol. 2003; 44:697–701.
4. Song HJ, Kim SJ. A study of antimicrobial sensitivity to the causative organism of urinary tract infection. Korean J Urol. 2005; 46:68–73.
5. Guidelines for ATC classification and DDD assignment. WHO;2005.
6. Kahlmeter G. An international survey of the antimicrobial susceptibility of pathogens from uncomplicated urinary tract infections: the ECO.SENS Project. J Antimicrob Chemother. 2003; 51:69–76.
7. Karlowsky JA, Kelly LJ, Thornsberry C, Jones ME, Sahm DF. Trends in antimicrobial resistance among urinary tract infection isolates of Escherichia coli from female outpatients in the United States. Antimicrob Agents Chemother. 2002; 46:2540–5.
8. Kim SW, Lee JY, Park WJ, Cho Y, Yoon MS. Antibiotic sensitivity to the causative organism of acute simple urinary tract infection. Korean J Urol. 2000; 41:1117–24.
9. Naber KG. Use of quinolones in urinary tract infections and prostatitis. Rev Infect Dis. 1989; 11(Suppl 5):S1321–37.

10. Sohn DW, Ha US, Seo HJ, Lee JY, Kim SW, Cho YH. In-vitro susceptibility study of oral antibiotics to Escheria coli, isolated from acute uncomplicated cystitis in female outpatients. Infect Chemother. 2006; 38:140–5.
11. Ryu KH, Kim MK, Jeong YB. A recent study on the antimicrobial sensitivity of the organisms that cause urinary tract infection. Korean J Urol. 2007; 48:638–45.

12. Karaca Y, Coplu N, Gozalan A, Oncul O, Citil BE, Esen B. Co-trimoxazole and quinolone resistance in Escherichia coli isolated from urinary tract infections over the last 10 years. Int J Antimicrob Agents. 2005; 26:75–7.

13. Goettsch W, van Pelt W, Nagelkerke N, Hendrix MG, Buiting AG, Petit PL, et al. Increasing resistance to fluoroquinolones in escherichia coli from urinary tract infections in the netherlands. J Antimicrob Chemother. 2000; 46:223–8.

14. Alos JI, Serrano MG, Gomez-Garces JL, Perianes J. Antibiotic resistance of Escherichia coli from community-acquired urinary tract infections in relation to demographic and clinical data. Clin Microbiol Infect. 2005; 11:199–203.
15. Monnet DL, Archibald LK, Phillips L, Tenover FC, McGowan JE Jr, Gaynes RP. Antimicrobial use and resistance in eight US hospitals: complexities of analysis and modeling. Intensive Care Antimicrobial Resistance Epidemiology Project and National Nosocomial Infections Surveillance System Hospitals. Infect Control Hosp Epidemiol. 1998; 19:388–94.
16. Mouton RP, Hermans J, Simoons-Smit AM, Hoogkamp-Korstanje JA, Degener JE, van Klingeren B. Correlations between consumption of antibiotics and methicillin resistance in coagulase negative staphylococci. J Antimicrob Chemother. 1990; 26:573–83.

17. Shlaes DM, Gerding DN, John JF Jr, Craig WA, Bornstein DL, Duncan RA, et al. Society for Healthcare Epidemiology of America and Infectious Diseases Society of America Joint Committee on the Prevention of Antimicrobial Resistance: guidelines for the prevention of antimicrobial resistance in hospitals. Infect Control Hosp Epidemiol. 1997; 18:275–91.

Go to : 
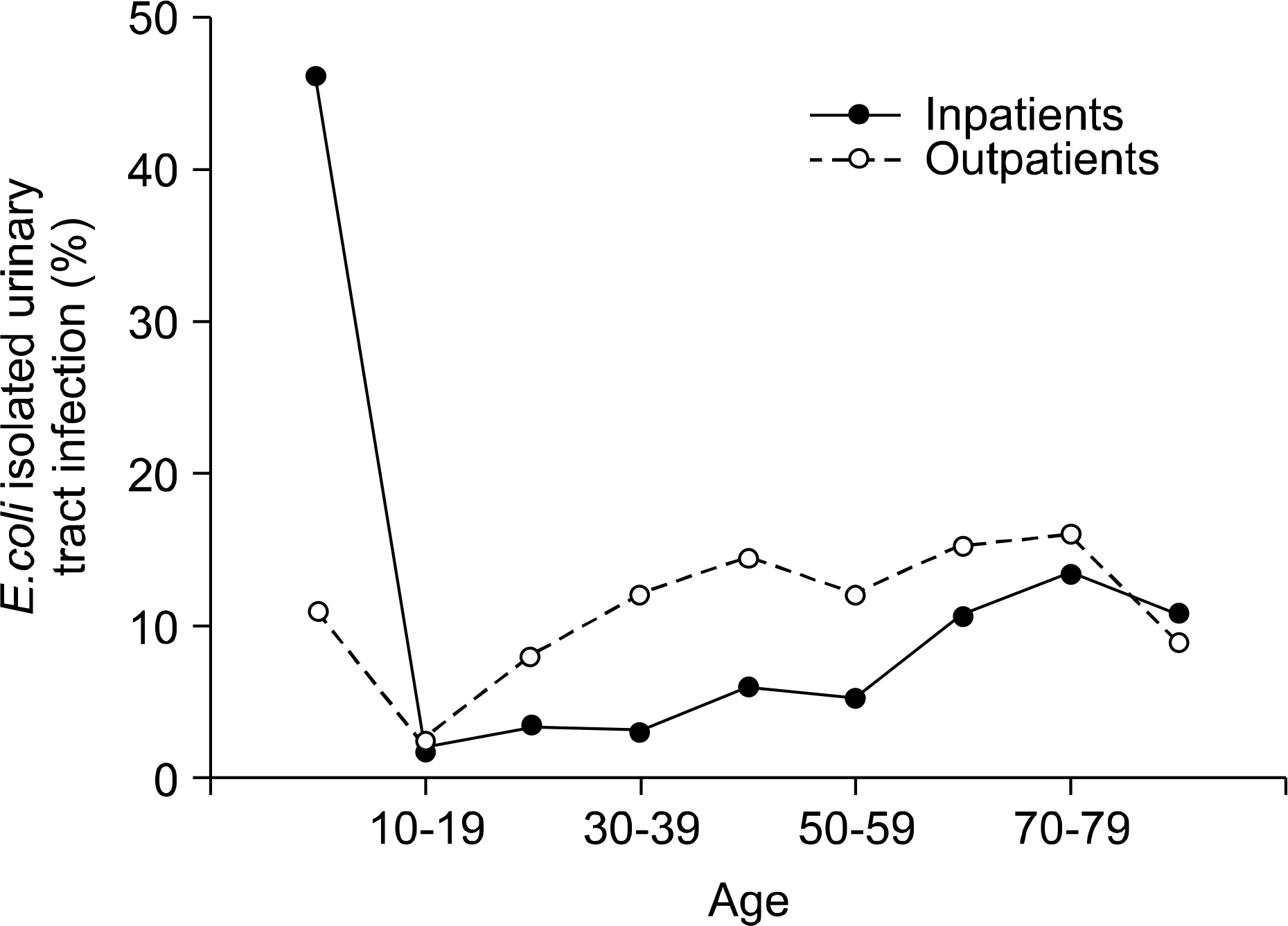 | Fig. 1.Age distribution according to the inpatients and outpatients with E.coli isolated urinary tract infection. |
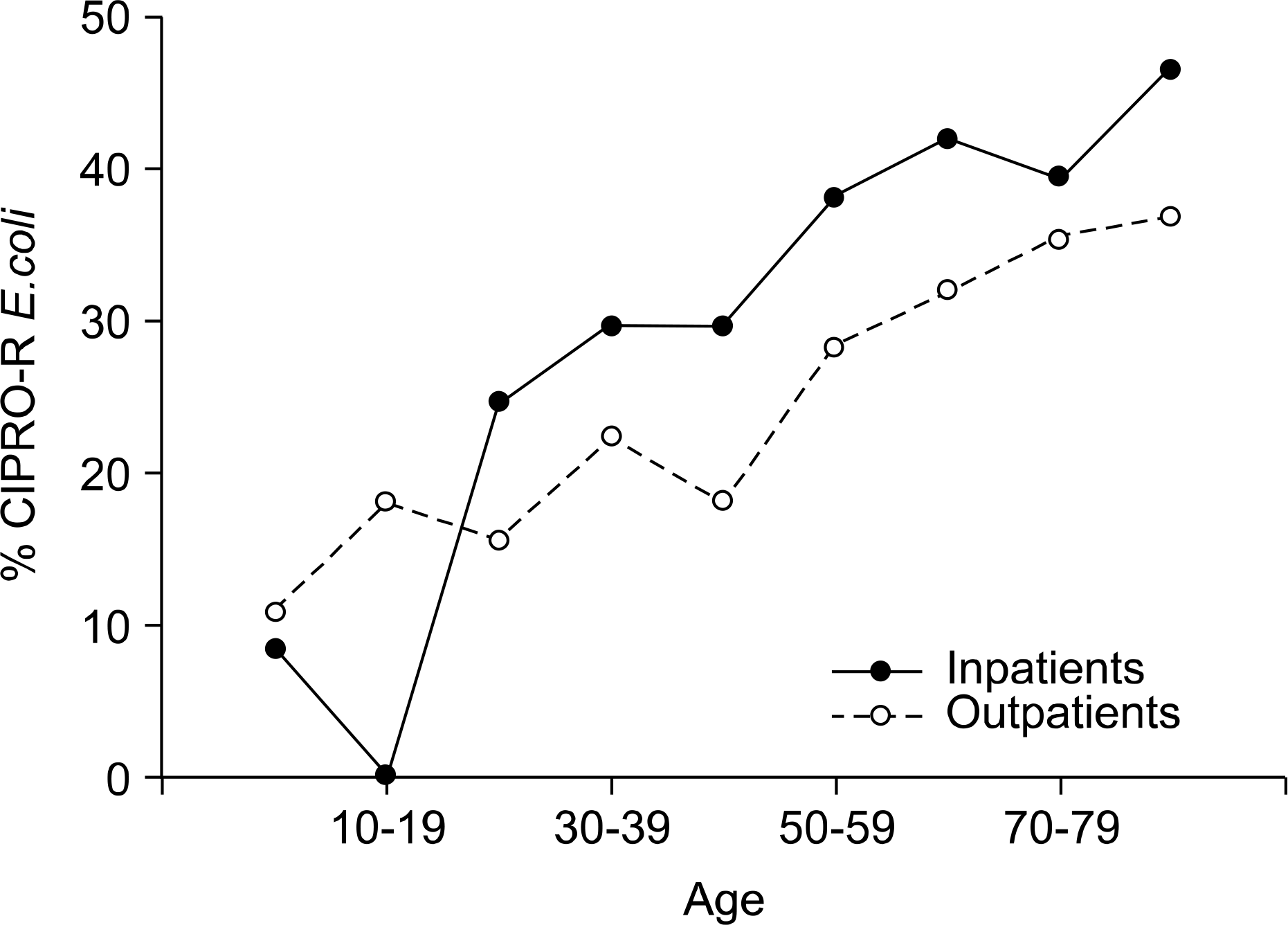 | Fig. 2.Age distribution according to the inpatients and outpatients and the rates of isolating ciprofloxacin‐resistant (CIPRO-R) E.coli in urinary tract infection. |
Table 1.
Common diseases of E.coli induced urinary tract infectio in inpatients and outpatients and the rates of isolating CIPRO‐ E.coli
Table 2.
The rates of isolating CIPRO-R E.coli and the AUD of quinolone according to the year
Table 3.
Multivariate analysis of the association of the risk factors with CIPRO-R E.coli urinary tract infection in the inpatients
Table 4.
Sensitivity to other antibiotics by CIPRO-R E.coli in the inpatients and outpatients




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download