Abstract
Purpose
The overuse of ciprofloxacin has recently increased the resistance of the Escherichia coli (E. coli). We studied the prevalence od the ciprofloxacin-resistant (CR) E. coli that were isolated from female patients with community-acquired urinary tract infection (CAUTI), and we demonstrated the resistant rate to other antibiotics to help physicians choose the suitable antibiotics to properly treat CAUTI.
Results
The incidence of UTI by E. coli increased with age (p<0.001), and it was highest in the 7th decade (59.0%). One hundred seventeen (30.2%) patients showed ciprofloxacin resistance. It was significantly related to an increased age (p=0.034), complicated UTI (p=0.04) and a previous history of antibiotic use (p=0.023). Trimethoprim/sulfamethoxazole (TMP/SMX) and fosfomycin showed similar resistance rates like ciprofloxacin; 31.8 and 28.2%, respectively. On the other hand, nitrofurantoin showed a low resistant rate of 5.7%. The resistance to cephalosporin was low in general; the lowest was cefepime (5.9%).
Conclusions
Our results imply that the empirical use of ciprofloxacin for female patients with CAUTI is questionable, and especially for patients older than 40 years old, patients with complicated UTI and patients with a previous history of antibiotic use. Nitrofurantoin and cephalosporin can be useful agents for the treatment of female CAUTI.
Go to : 
REFERENCES
1. Warren JW, Abrutyn E, Hebel JR, Johnson JR, Schaeffer AJ, Stamm WE. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA). Clin Infect Dis. 1999; 29:745–58.
2. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002; 113(Suppl 1A):S5–13.

3. Kunin CM. An overview of urinary tract infection. Definition, prevention and management. 5th ed.Baltimore: Williams & Wilkins;1998. p. 2–21.
4. Engel JD, Schaeffer AJ. Evaluation of and antimicrobial therapy for recurrent urinary tract infections in women. Urol Clin North Am. 1998; 25:685–701.

5. Stamm WE, Norrby SR. Urinary tract infections: disease panorama and challenges. J Infect Dis. 2001; 183(Suppl 1):S1–4.

6. Nicolle LE. Epidemiology of urinary infection. Infect Med. 2001; 18:153–62.
7. Song HJ, Kim SJ. A study of antimicrobial sensivity to the causative organism of urinary tract infection. Korean J Urol. 2005; 46:68–73.
8. Ryu KH, Kim MK, Jeong YB. A recent study on the antimicrobial sensitivity of the organisms that cause urinary tract infection. Korean J Urol. 2007; 48:638–45.

9. Ti TY, Kumarasinghe G, Taylor MB, Tan SL, Ee A, Chua C, et al. What is true community-acquired urinary tract infection? Comparison of pathogens identified in urine from routine outpatient specimens and from community clinics in a prospective study. Eur J Clin Microbiol Infect Dis. 2003; 22:242–5.

10. Chang IH, Bang SH, Choi NY, Park SY, Han JH, Ahn SH. Trends in the emergence of ciprofloxacin-resistant escherichia coli and the relationship with underlying diseases in patients with urinary tract infection. Korean J Urol. 2008; 49:66–71.

11. Lee SJ, Cho YH, Kim BW, Lee JG, Jung SI, Lee SD, et al. A multicenter study of antimicrobial susceptibility of uro-pathogens causing acute uncomplicated cystitis in woman. Korean J Urol. 2003; 44:697–701.
12. Ko YH, Oh JS, Cho DY, Bea JH, Koh SK. Changes of causative organisms and antimicrobial sensitivity of urinary tract infection between 1979 and 2001. Korean J Urol. 2003; 44:342–50.
13. Karlowsky JA, Kelly LJ, Thornsberry C, Jones ME, Sahm DF. Trends in antimicrobial resistance among urinary tract infection isolates of Escherichia coli from female outpatient in the United States. Antimicrob Agents Chemother. 2002; 46:2540–5.
14. Tice AD. Short-course therapy of acute cystitis: a brief review of therapeutic strategies. J Antimicrob Chemother. 1999; 43(Suppl A):85–93.
15. Karaca Y, Coplu N, Gozalan A, Oncul O, Citil BE, Esen B. Co-trimoxazole and quinolone resistance in Escherichia coli isolated from urinary tract infections over the last 10 years. Int J Antimicrob Agents. 2005; 26:75–7.

16. Gobernado M, Valdes L, Alos JI, Garcia-Rey C, Dal-Re R, Garcia-de-Lomas J, et al. Quinolone resistance in female outpatient urinary tract isolates of Escherichia coli: age-related differences. Rev Esp Quimioter. 2007; 20:206–10.
17. Hames L, Rice CE. Antimicrobial resistance of urinary tract isolates in acute uncomplicated cystitis among college-aged women: choosing a first-line therapy. J Am Coll Health. 2007; 56:153–6.

18. Wright SW, Wrenn KD, Haynes ML. Trimethoprim-sulfamethoxazole resistance among urinary coliform isolates. J Gen Intern Med. 1999; 14:606–9.

19. Neuman M, Fluteau G. Treatment of urinary infections with fosfomycin. Chemotherapy. 1977; 23(Suppl 1):259–64.

20. Lee SE, Choi H, Kim YK. A clinical study of oral fosfomycin (FosmyinⓇ) in the treatment of lower urinary tract infection. Korean J Urol. 1984; 25:167–72.
21. Lobel B, Valot A, Cattoir V, Lemenand O, Gaillot O. Comparison of antimicrobial susceptibility of 1,217 Escherichia coli isolates from women with hospital and community-acquired urinary tract infections. Presse Med. 2008; 37:746–50.
22. Tullio V, Cuffini AM, Banche G, Mandras N, Allizond V, Roana J, et al. Role of fosfomycin tromethamine in modulating non-specific defence mechanisms in chronic uremic patients towards ESBL-producing Escherichia coli. Int J Immunopathol Pharmacol. 2008; 21:153–60.

23. Mathai E, Chandy S, Thomas K, Antoniswamy B, Joseph I, Mathai M, et al. Antimicrobial resistance surveillance among commensal Escherichia coli in rural and urban areas in Southern India. Trop Med Int Health. 2008; 13:41–5.

24. Junquera S, Loza E, Baquero F. Changes in the antimicrobial susceptibility of Escherichia coli isolates from nosocomial versus community-acquired urinary tract infections. Enferm Infecc Microbiol Clin. 2005; 23:197–201.
Go to : 
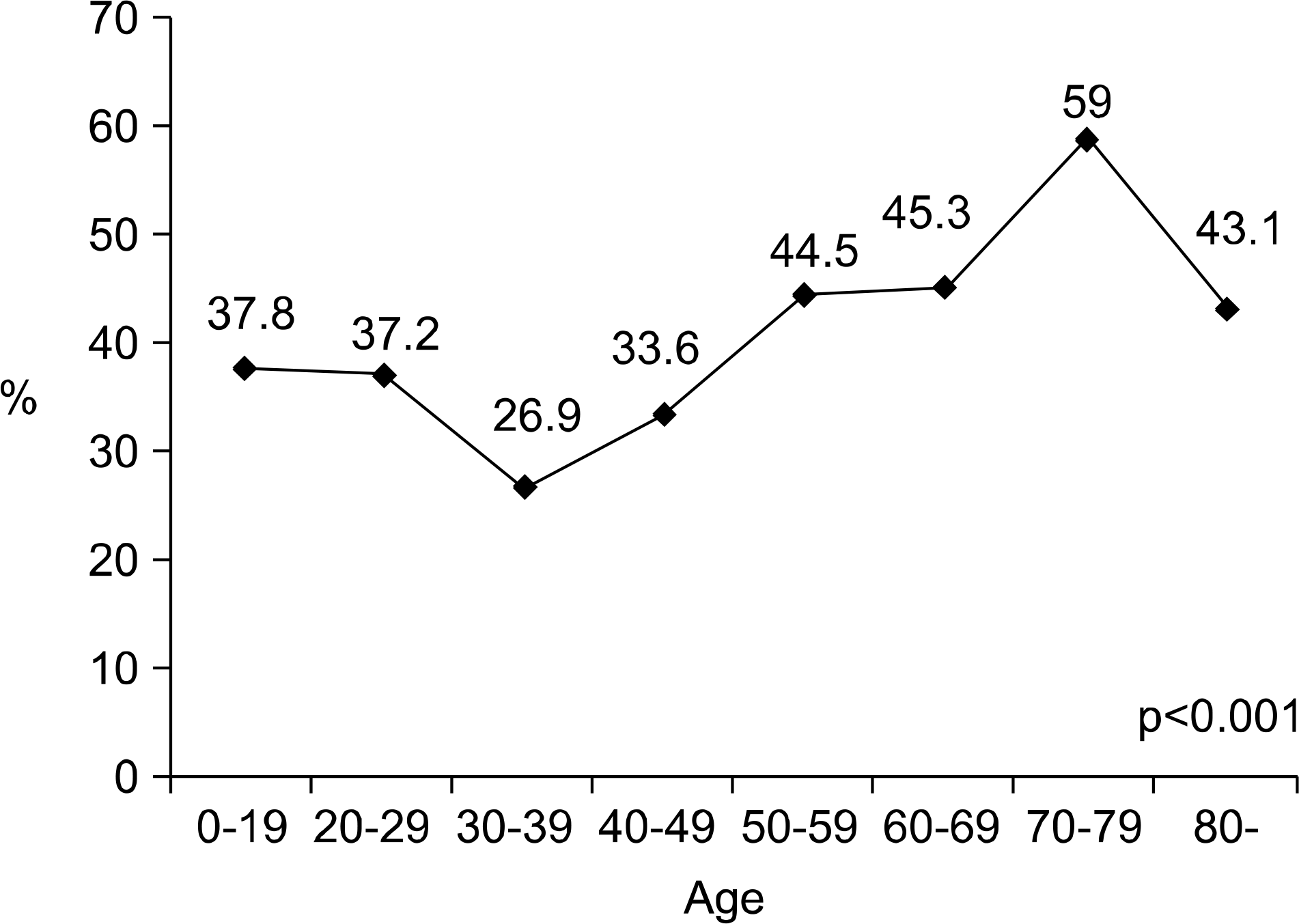 | Fig. 1.Age-specific incidence of the E. coli induced female CAUTI. CAUTI: community-acquired urinary tract infection. |
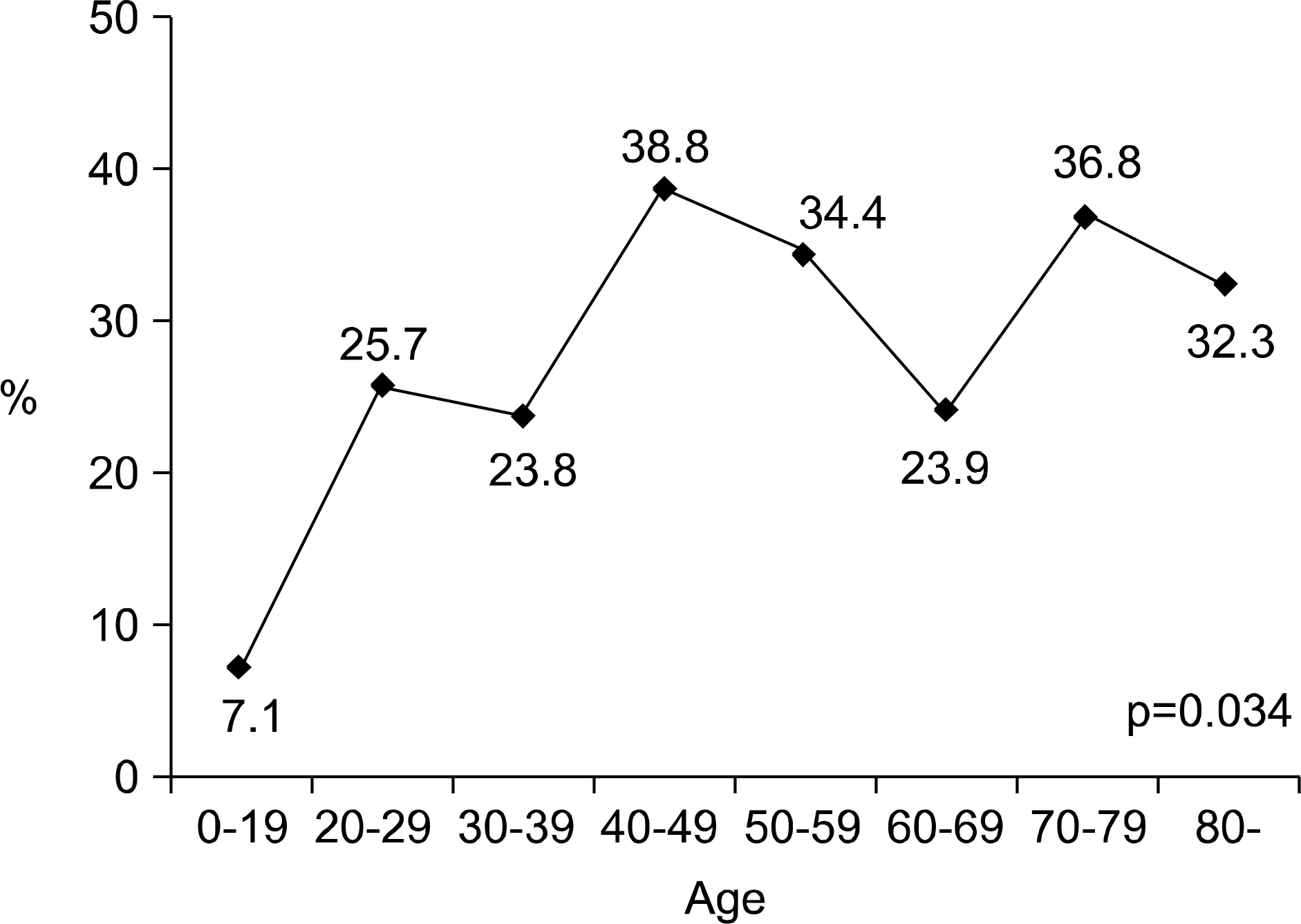 | Fig. 2.Age-specific resistant rate to ciprofloxacin of the E. coli isolated from female patients with CAUTI. CAUTI: community-acquired urinary tract infection. |
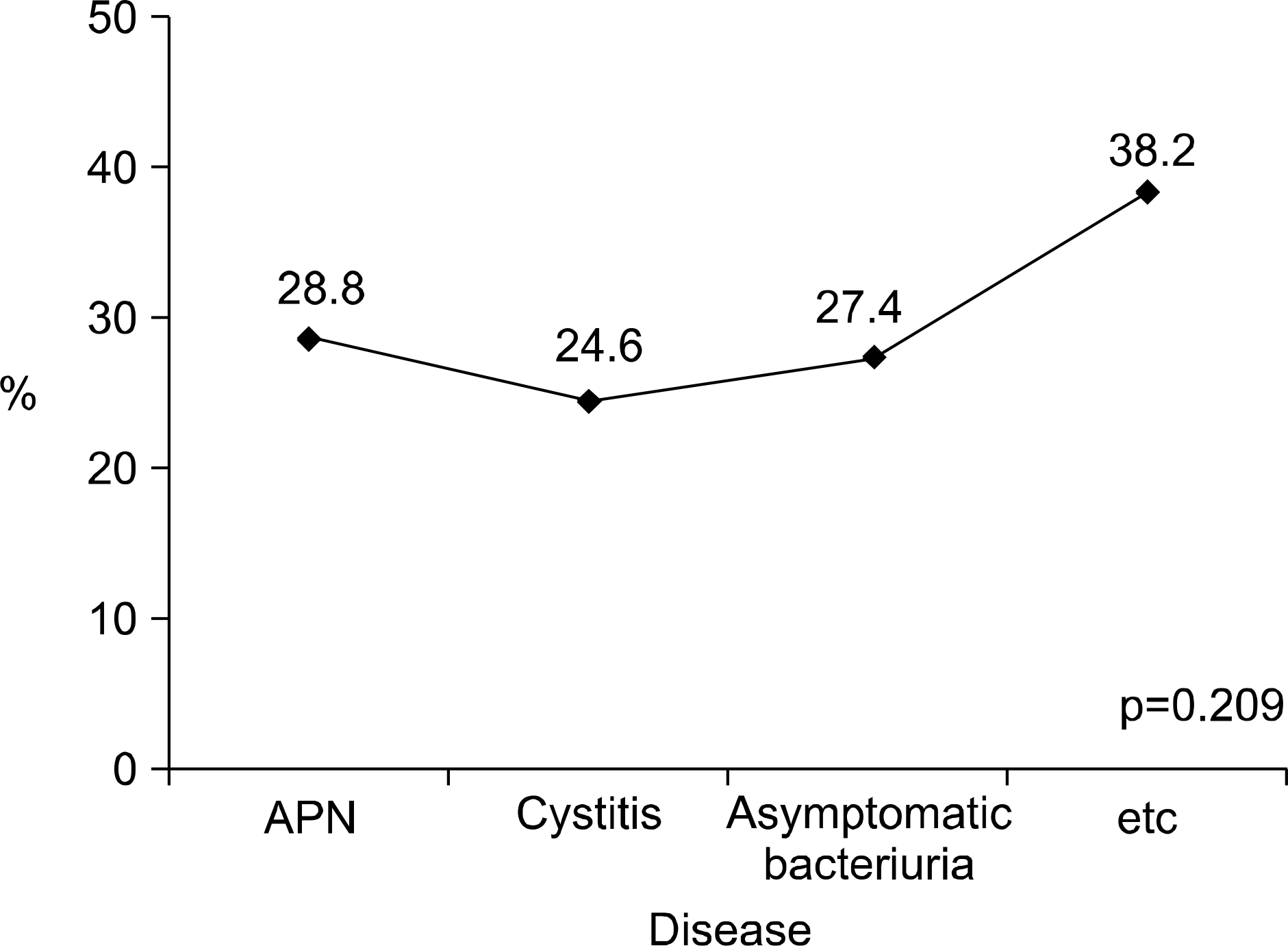 | Fig. 3.Disease-specific resistant rate to ciprofloxacin of the E. coli isolated from female patients with CAUTI. APN: acute pyelonephritis, CAUTI: community-acquired urinary tract infection. |
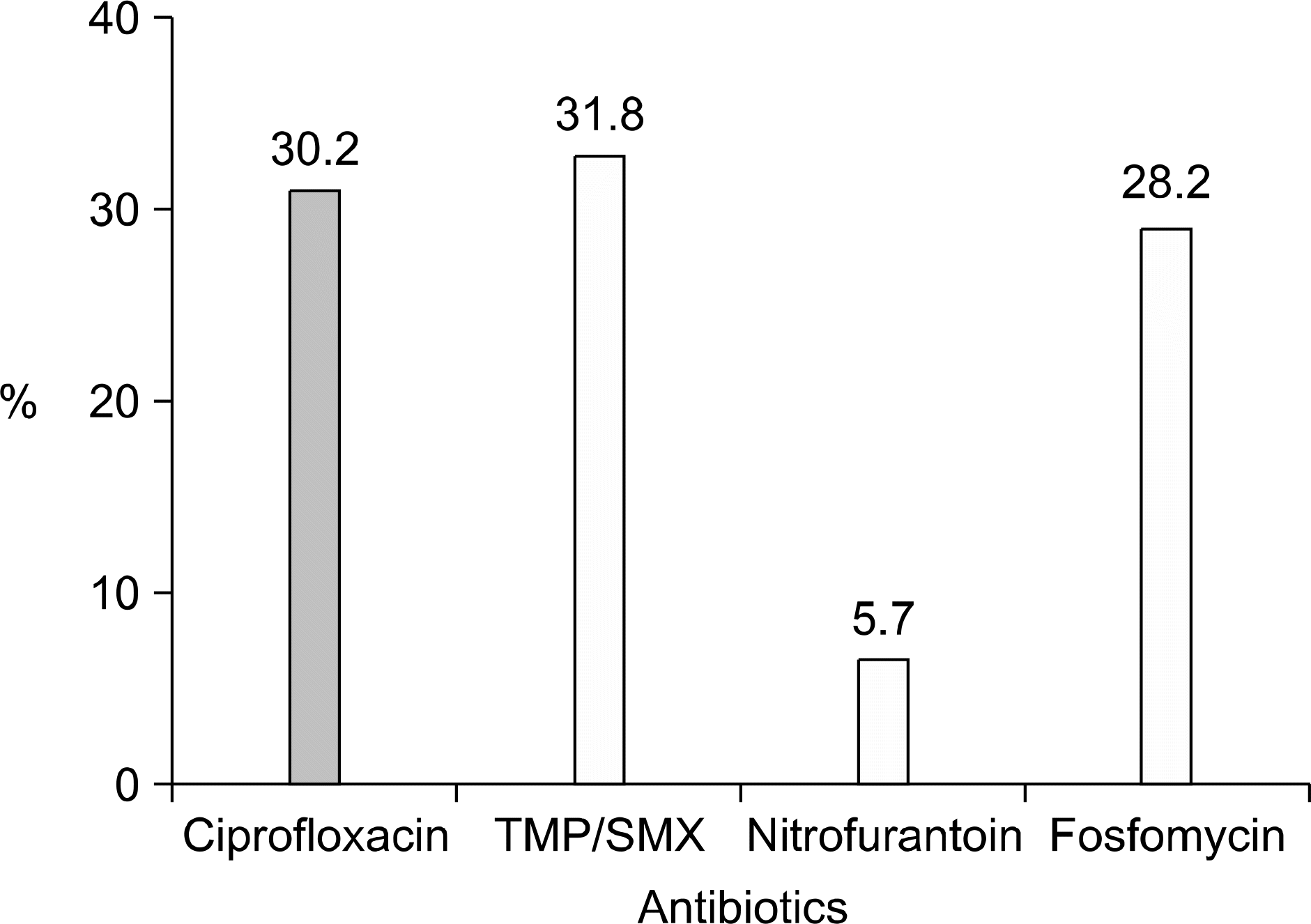 | Fig. 4.Resistant rate of the E. coli isolated from female patients with CAUTI to the antibiotics recommended by the IDSA guidelines. TMP/SMX: trimethoprim/sulfamethoxazole, IDSA: infectious diseases society of America, CAUTI: community-acquired urinary tract infection. |
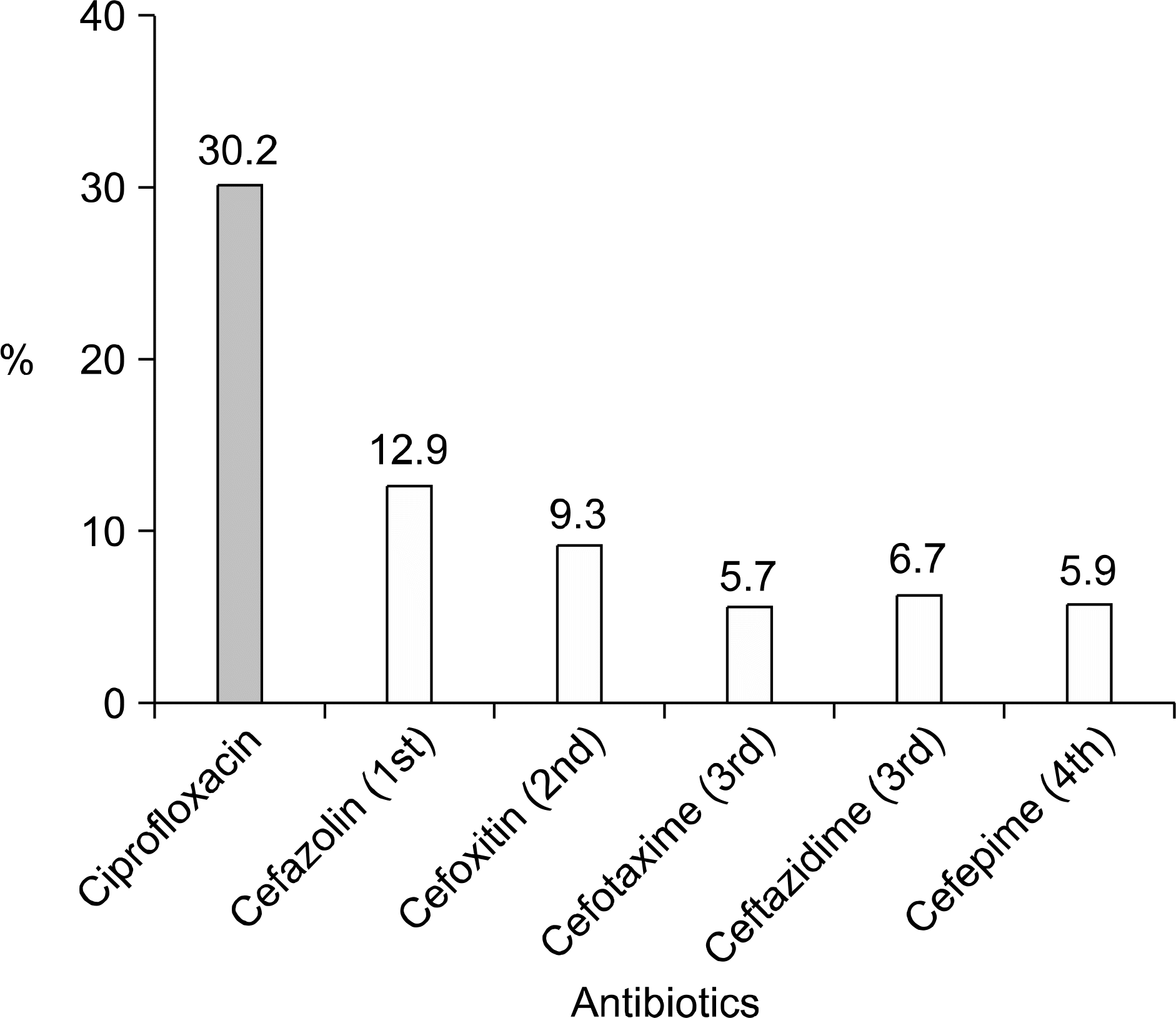 | Fig. 5.Resistant rate to cephalosporin of the E. coli isolated from female patients with CAUTI. CAUTI: community-acquired urinary tract infection. |
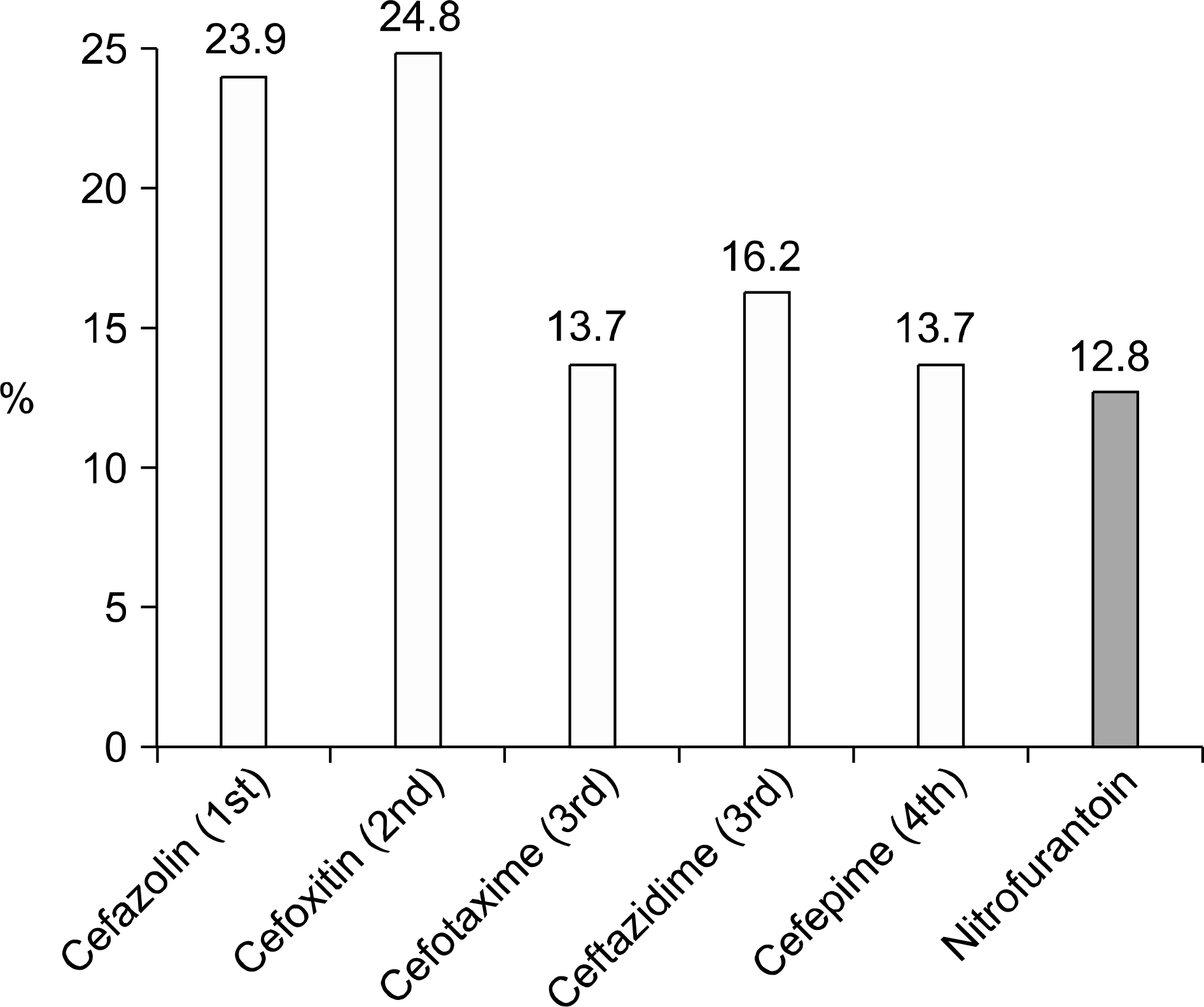 | Fig. 6.Resistant rate to cephalosporin and nitrofurantoin of the ciprofloxacin-resistant E. coli isolated from female patients with CAUTI. CAUTI: community-acquired urinary tract infection. |
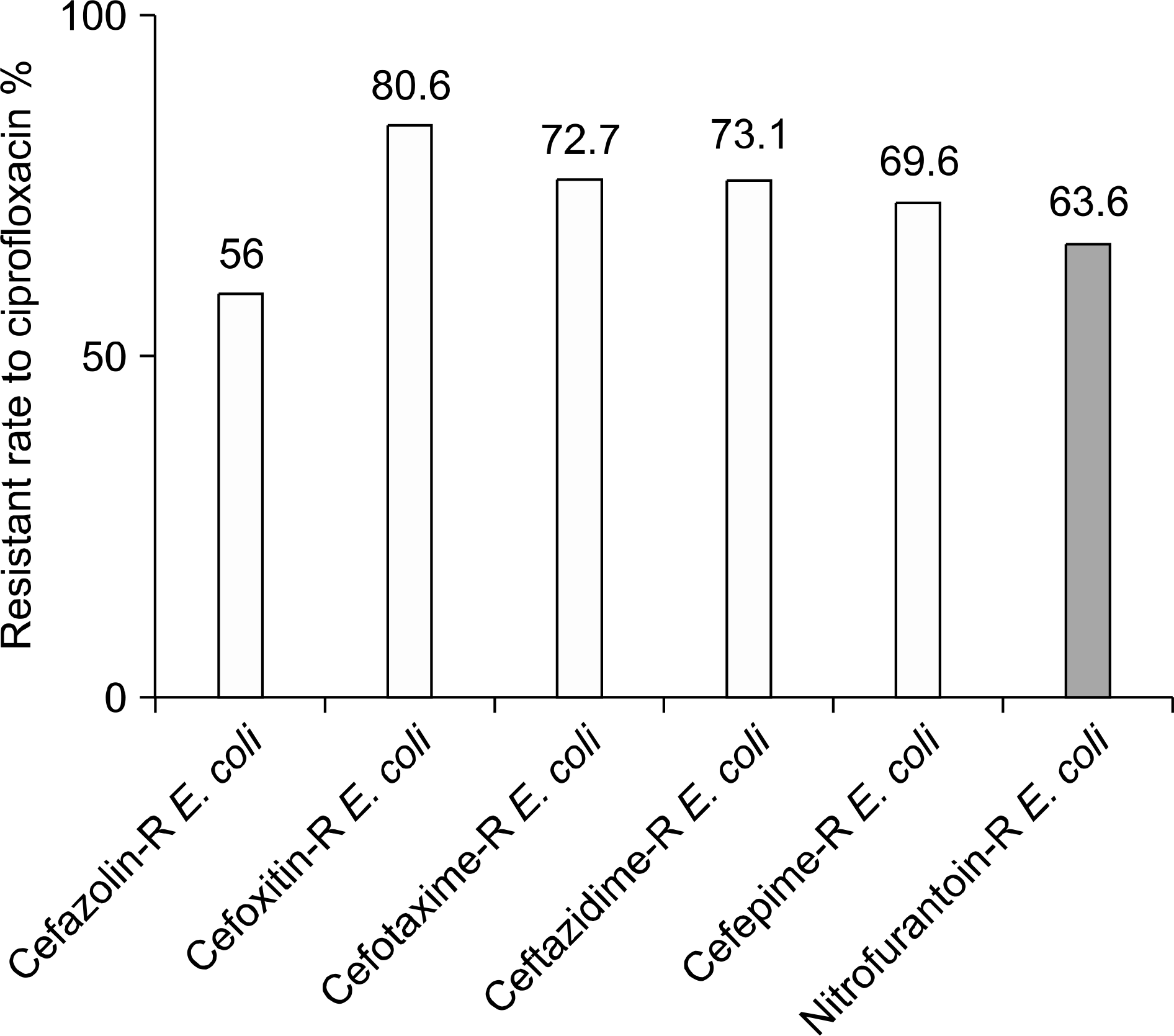 | Fig. 7.Resistant rate to ciprofloxacin of the cephalosporin-resistant and nitrofurantoin-resistant E. coli isolated from female patients with CAUTI. R: resistant, CAUTI: community-acquired urinary tract infection. |
Table 1.
Age-specific distribution of the ciprofloxacin-sensitive E. coli and ciprofloxacin-resistant E. coli according to the disease causing the community-acquired urinary tract infection in females
Table 2.
Association of the previously (recent 1 year) used antibiotics with isolating ciprofloxacin-resistant E. coli
| Previous antibiotics use (recent 1 year) | CR E. coli | CS E. coli | Total |
|---|---|---|---|
| Yes (%) | 63 (36.4) | 110 (63.6) | 173 (100) |
| No (%) | 54 (25.2) | 160 (74.8) | 214 (100) |
| Total (%) | 117 (30.2) | 270 (69.8) | 387 (100) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download