Abstract
Purpose
Amplification and mutation of the epidermal growth factor receptor (EGFR) and HER-2 genes were analyzed in the tissues of hormone refractory prostate cancer (HRPC) patients.
Materials and Methods
Gene amplifications of the EGFR and HER-2 gene were analyzed by fluorescence in situ hybridization (FISH) with direct sequencing. Studies were performed on 10 patients; tissues were sampled at the time of initial diagnosis and after the conversion to HRPC (a total of 20 tissue samples). Direct sequencing was performed on exons 18–24 of the EGFR gene and exons 19 and 20 of the HER-2 gene. The amplifications and mutations were compared with the clinicopathologic features.
Results
Gene amplification of the EGFR gene was observed in 6 (30%) out of 20 samples. A total of six EGFR mutations in exons 18 and 19 were detected in three pairs of tissues (three patients). One patient with a hormone refractory status had a novel deletion mutation in EGFR exon 19. EGFR mutations were associated with the acinar type of prostate cancer, but they were not associated with the ductal type. No significant correlation was found between mutation change and the hormone sensitive or refractory status. However, the time to convert to HRPC was significantly shorter in the patients with a mutation in the EGFR gene (p=0.017). There were no HER-2 gene amplifications or mutations found in any of the samples.
References
1. So A, Gleave M, Hurtado-Col A, Nelson C. Mechanisms of the development of androgen independence in prostate cancer. World J Urol. 2005; 23:1–9.

2. Hellawell GO, Brewster SF. Growth factors and their receptors in prostate cancer. BJU Int. 2002; 89:230–40.

3. Bartlett JM, Brawley D, Grigor K, Munro AF, Dunne B, Edwards J. Type I receptor tyrosine kinases are associated with hormone escape in prostate cancer. J Pathol. 2005; 205:522–9.

4. Di Lorenzo G, Tortora G, D'Armiento FP, De Rosa G, Staibano S, Autorino R, et al. Expression of epidermal growth factor receptor correlates with disease relapse and progression to androgen-independence in human prostate cancer. Clin Cancer Res. 2002; 8:3438–44.
5. Hernes E, Fossa SD, Berner A, Otnes B, Nesland JM. Expression of the epidermal growth factor receptor family in prostate carcinoma before and during androgen-independence. Br J Cancer. 2004; 90:449–54.

6. Osman I, Scher HI, Drobnjak M, Verbel D, Morris M, Agus D, et al. HER-2/neu (p185neu) protein expression in the natural or treated history of prostate cancer. Clin Cancer Res. 2001; 7:2643–7.
7. Shi Y, Brands FH, Chatterjee S, Feng AC, Groshen S, Schewe J, et al. Her-2/neu expression in prostate cancer: high level of expression associated with exposure to hormone therapy and androgen independent disease. J Urol. 2001; 166:1514–9.

8. Signoretti S, Montironi R, Manola J, Altimari A, Tam C, Bubley G, et al. Her-2-neu expression and progression toward androgen independence in human prostate cancer. J Natl Cancer Inst. 2000; 92:1918–25.

9. Canil CM, Moore MJ, Winquist E, Baetz T, Pollak M, Chi KN, et al. Randomized phase II study of two doses of gefitinib in hormone-refractory prostate cancer: a trial of the National Cancer Institute of Canada-Clinical Trials Group. J Clin Oncol. 2005; 23:455–60.

10. Formento P, Hannoun-Levi JM, Fischel JL, Magne N, Etienne-Grimaldi MC, Milano G. Dual HER 1–2 targeting of hormone-refractory prostate cancer by ZD1839 and trastuzumab. Eur J Cancer. 2004; 40:2837–44.

11. Sgambato A, Camerini A, Faraglia B, Ardito R, Bianchino G, Spada D, et al. Targeted inhibition of the epidermal growth factor receptor-tyrosine kinase by ZD1839 (‘Iressa') induces cell-cycle arrest and inhibits proliferation in prostate cancer cells. J Cell Physiol. 2004; 201:97–105.

12. Arteaga CL. Epidermal growth factor receptor dependence in human tumors: more than just expression? Oncologist. 2002; 7(Suppl 4):31–9.

13. Cappuzzo F, Hirsch FR, Rossi E, Bartolini S, Ceresoli GL, Bemis L, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005; 97:643–55.

14. Riely GJ, Pao W, Pham D, Li AR, Rizvi N, Venkatraman ES, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2006; 12:839–44.

15. Janne PA, Borras AM, Kuang Y, Rogers AM, Joshi VA, Liyanage H, et al. A rapid and sensitive enzymatic method for epidermal growth factor receptor mutation screening. Clin Cancer Res. 2006; 12:751–8.

16. Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005; 97:339–46.

17. Skacel M, Ormsby AH, Pettay JD, Tsiftsakis EK, Liou LS, Klein EA, et al. Aneusomy of chromosomes 7, 8, and 17 and amplification of HER-2/neu and epidermal growth factor receptor in Gleason score 7 prostate carcinoma: a differential fluorescent in situ hybridization study of Gleason pattern 3 and 4 using tissue microarray. Hum Pathol. 2001; 32:1392–7.

18. Cui J, Deubler DA, Rohr LR, Zhu XL, Maxwell TM, Changus JE, et al. Chromosome 7 abnormalities in prostate cancer detected by dual-color fluorescence in situ hybridization. Cancer Genet Cytogenet. 1998; 107:51–60.

19. Bubendorf L, Kononen J, Koivisto P, Schraml P, Moch H, Gasser TC, et al. Survey of gene amplifications during prostate cancer progression by high-throughout fluorescence in situ hybridization on tissue microarrays. Cancer Res. 1999; 59:803–6.
20. Kaltz-Wittmer C, Klenk U, Glaessgen A, Aust DE, Diebold J, Lohrs U, et al. FISH analysis of gene aberrations (MYC, CCND1, ERBB2, RB, and AR) in advanced prostatic carcinomas before and after androgen deprivation therapy. Lab Invest. 2000; 80:1455–64.

21. Ross JS, Sheehan CE, Hayner-Buchan AM, Ambros RA, Kallakury BV, Kaufman RP, et al. Prognostic significance of HER-2/neu gene amplification status by fluorescence in situ hybridization of prostate carcinoma. Cancer. 1997; 79:2162–70.

22. Tsao MS, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, Squire J, et al. Erlotinib in lung cancer – molecular and clinical predictors of outcome. N Engl J Med. 2005; 353:133–44.

23. Bellezza I, Bracarda S, Caserta C, Minelli A. Targeting of EGFR tyrosine kinase by ZD1839 (“Iressa”) in androgen-responsive prostate cancer in vitro. Mol Genet Metab. 2006; 88:114–22.

Fig. 1.
The change of the suppression of cell proliferation in response to epidermal growth factor (EGF) and EGF receptor (EGFR) tyrosine kinase inhibitor (EGFR-TKI) ZD1839 in a dose-dependent manner at the prostate cancer cell lines.
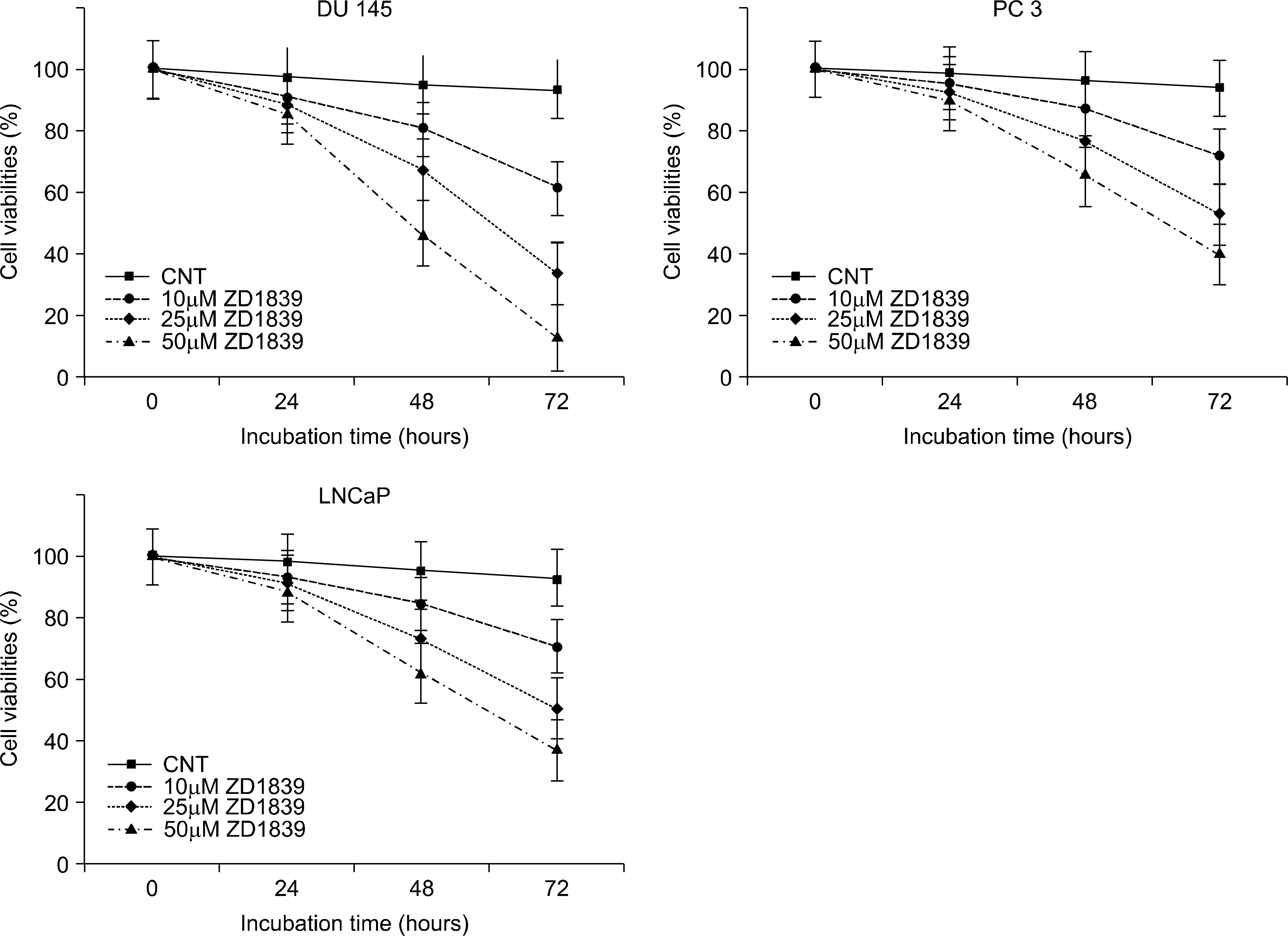
Fig. 2.
Observation of the amplification of the epidermal growth factor receptor (EGFR) gene by fluorescence in situ hybridization. Cells that were morphologically normal and had a ratio of the pink EGFR signal to the green centromere 7 signal higher than 2 in nonoverlapping nuclei were classified as having a gene amplification.
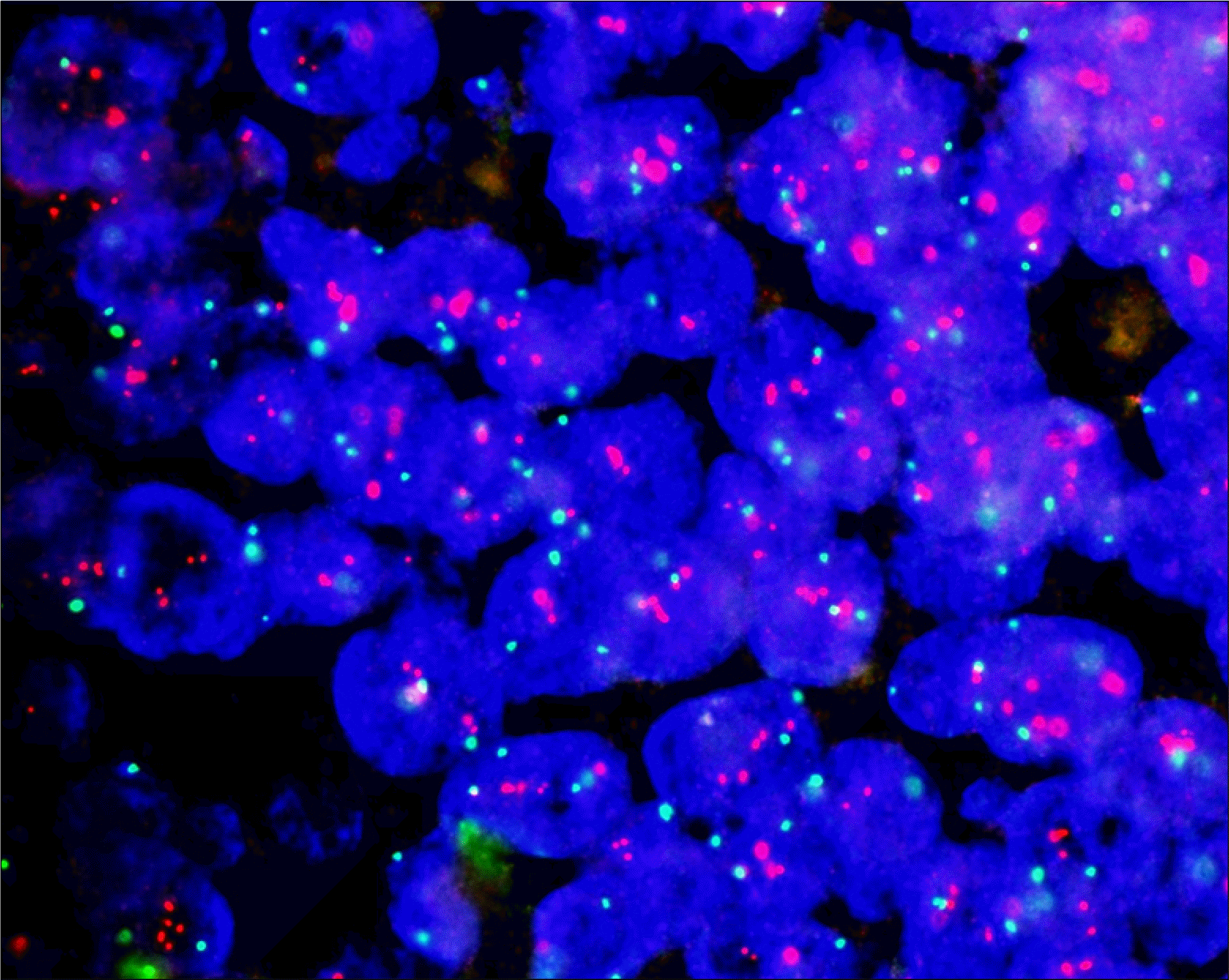
Table 1.
Clinical and pathological characteristic of the subject patients
Table 2.
The nucleic acid sequence of the forward and reverse primers for the EGFR and HER-2 genes
Table 3.
The summary of the amplification and mutations of the EGFR gene in acinar type adenocarcinoma




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download