Abstract
Purpose
We investigated the relationship of prostate volume with metabolic and anthropometric parameters in men who visited a health promotion center.
Materials and Methods
From January 2004 to July 2007, among 16,236 men between 30 to 69 years old who visited our health promotion center for a general check-up, 1,033 men (6.4%) agreed to have their prostate evaluation included in this study. They underwent anthropometric measurements, basic laboratory tests, and transrectal ultrasonography. We evaluated the relationship of prostate volume with metabolic and anthropometric factors.
Results
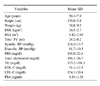
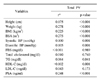
In bivariate analysis, prostate volume positively correlated with height, weight, body mass index (BMI), body surface area (BSA), serum prostate-specific antigen (PSA), triglyceride, and blood pressure, and negatively correlated with high-density lipoprotein cholesterol (HDL-C). In multivariate analysis, age, BSA, serum PSA and HDL-C significantly correlated with prostate volume, whereas BMI did not (p=0.765). Prostate volume in patients with metabolic syndrome (28.0ml) was significantly larger than those without (25.4ml), but there was no difference in PSA (p=0.976).
Figures and Tables
References
1. Ziada A, Rosenblum M, Crawford ED. Benign prostatic hyperplasia: an overview. Urology. 1999. 53:3 Suppl 3a. 1–6.
2. Lee C, Kozlowski JM, Grayhack JT. Etiology of benign prostatic hyperplasia. Urol Clin North Am. 1995. 22:237–246.
3. Partin AW, Oesterling JE, Epstein JI, Horton R, Walsh PC. Influence of age and endocrine factors on the volume of benign prostatic hyperplasia. J Urol. 1991. 145:405–409.
4. Dahle SE, Chokkalingam AP, Gao YT, Deng J, Stanczyk FZ, Hsing AW. Body size and serum levels of insulin and leptin in relation to the risk of benign prostatic hyperplasia. J Urol. 2002. 168:599–604.
5. Hammarsten J, Hogstedt B, Holthuis N, Mellstrom D. Components of the metabolic syndrome-risk factors for the development of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 1998. 1:157–162.
6. Giovannucci E, Rimm EB, Chute CG, Kawachi I, Colditz GA, Stampfer MJ, et al. Obesity and benign prostatic hyperplasia. Am J Epidemiol. 1994. 140:989–1002.
7. Gupta A, Gupta S, Pavuk M, Roehrborn CG. Anthropometric and metabolic factors and risk of benign prostatic hyperplasia: a prospective cohort study of Air Force veterans. Urology. 2006. 68:1198–1205.
8. Kim JH, Shim BS, Hong YS. The relating factors of metabolic syndrome to benign prostatic hyperplasia. Korean J Urol. 2005. 46:1046–1050.
9. Ozden C, Ozdal OL, Urgancioglu G, Koyuncu H, Gokkaya S, Memis A. The correlation between metabolic syndrome and prostatic growth in patients with benign prostatic hyperplasia. Eur Urol. 2007. 51:199–203.
10. Park JS, Park JK. The meaning of metabolic syndrome X in patients suffering with benign prostatic hyperplasia. Korean J Urol. 2007. 8:696–700.
11. Sarma AV, Jaffe CA, Schottenfeld D, Dunn R, Montie JE, Cooney KA, et al. Insulin-like growth factor-1, insulin-like growth factor binding protein-3, and body mass index: clinical correlates of prostate volume among black men. Urology. 2002. 59:362–367.
12. Xie LP, Bai Y, Zhang XZ, Zheng XY, Yao KS, Xu L, et al. Obesity and benign prostatic enlargement: a large observational study in China. Urology. 2007. 69:680–684.
13. Sohn JC, Chang HS, Kim CI. The correlation between metabolic syndrome and the prostate volume. Korean J Urol. 2007. 48:603–607.
14. Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Arch Intern Med. 1916. 17:863–871.
15. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001. 285:2486–2497.
16. Western Pacific Regional Office of the World Health Organization, the International Obesity Task Force. The Asia-Pacific perspective: redefining obesity and its treamtnet. 2000. Sydney: Health Communications Australia;
http://www.obesityasiapacific.com
.
17. Roberts RO, Jacobson DJ, Rhodes T, Klee GG, Leiber MM, Jacobsen SJ. Serum sex hormones and measures of benign prostatic hyperplasia. Prostate. 2004. 61:124–131.
18. Pasquali R, Casimirri F, Cantobelli S, Melchionda N, Morselli Labate AM, Fabbri R, et al. Effect of obesity and body fat distribution on sex hormones and insulin in men. Metabolism. 1991. 40:101–104.
19. Wrana JL, Attisano L, Wieser R, Ventura F, Massague J. Mechanism of activation of the TGF-beta receptor. Nature. 1994. 370:341–347.
20. Landsberg L. Diet, obesity and hypertension: an hypothesis involving insulin, the sympathetic nervous system, and adaptive thermogenesis. Q J Med. 1986. 61:1081–1090.
21. Peehl DM, Cohen P, Rosenfeld RG. The role of insulin-like growth factors in prostate biology. J Androl. 1996. 17:2–4.
22. Torring N, Vinter-Jensen L, Pedersen SB, Sorensen FB, Flyvbjerg A, Nexo E. Systemic administration of insulin-like growth factor I (IGF-I) causes growth of the rat prostate. J Urol. 1997. 158:222–227.
23. Lim S, Park KS, Lee HK, Cho SI. Changes in the characteristics of metabolic syndrome in Korea over the period 1998-2001 as determined by Korean National Health and Nutrition Examination Surveys. Diabetes Care. 2005. 28:1810–1812.
24. Parsons JK, Carter HB, Partin AW, Windham BG, Metter EJ, Ferrucci L, et al. Metabolic factors associated with benign prostatic hyperplasia. J Clin Endocrinol Metab. 2006. 91:2562–2568.
25. Signorello LB, Tzonou A, Lagiou P, Samoli E, Zavitsanos X, Trichopoulos D. The epidemiology of benign prostatic hyperplasia: a study in Greece. BJU Int. 1999. 84:286–291.
26. Barnard RJ, Aronson WJ, Tymchuk CN, Ngo TH. Prostate cancer: another aspect of the insulin-resistance syndrome? Obes Rev. 2002. 3:303–308.
27. Ochiai A, Fritsche HA, Babaian RJ. Influence of anthropometric measurements, age, and prostate volume on prostate-specific antigen levels in men with a low risk of prostate cancer. Urology. 2005. 66:819–823.
28. Lee S, Min HG, Choi SH, Kim YJ, Oh SW, Kim YJ, et al. Central obesity as a risk factor for prostatic hyperplasia. Obesity. 2006. 14:172–179.
29. Rohrmann S, Smit E, Giovannucci E, Platz EA. Association between markers of the metabolic syndrome and lower urinary tract symptoms in the Third National Health and Nutrition Examination Survey (NHANES III). Int J Obes (Lond). 2005. 29:310–316.
30. Bañez LL, Hamilton RJ, Vollmer RT, Moul JW, Amling CL, Kane CJ, et al. Can hemodilution explain the lower PSA concentrations among obese men? J Urol. 2007. 177:Suppl. 468. abstract 1418.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download