Abstract
Purpose
The purpose of this study was to evaluate the effects of estrogen on detrusor contraction and the expression of muscarinic receptors in ovariectomized rats.
Materials and Methods
24 Sprague-Dawley female virgin rats (12 weeks old) were separated into three groups of 8 rats each. Group I served as a control group, group II was the ovariectomized only rats (Ovx group) and Group III was given estradiol benzoate (0.8mg/kg/day) subcutaneously for 7 consecutive days, beginning 1 week after ovariectomy (Ovx+E group). At the end of the experimental period, each rat was sacrificed and the urinary bladder was removed for contractile studies. The expressions of M2 and M3 receptors in the bladder epithelium and the muscle layer were investigated by performing immunofluorescent staining.
Results
The Ovx group showed a significantly decreased bladder contractile function on the KCl and carbachol-induced contractile tests, whereas the Ovx+E group showed increased contractility (p<0.05). The Ovx+E group showed an increase of smooth muscle compared to the other groups. Ovariectomy induced a significant increase in the M3 receptors density in the bladder body, as compared to the control group (p<0.05) but there was no significant difference between the Ovx group and the Ovx+E group.
Go to : 
References
1. Keane DP, O'Sullivan S. Urinary incontinence: anatomy, physiology and pathophysiology. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000; 14:207–26.

2. Lin AD, Levin R, Kogan B, Whitbeck C, Chichester P, Sokol R, et al. Estrogen induced functional hypertrophy and increased force generation of the female rabbit bladder. Neurourol Urodyn. 2006; 25:473–9.

3. Simunic V, Banovic I, Ciglar S, Jeren L, Pavicic Baldani D, Sprem M. Local estrogen treatment in patients with urogenital symptoms. Int J Gynaecol Obstet. 2003; 82:187–97.
4. Zullo MA, Plotti F, Calcagno M, Palaia I, Muzii L, Manci N, et al. Vaginal estrogen therapy and overactive bladder symptoms in postmenopausal patients after a tension-free vaginal tape procedure: a randomized clinical trial. Menopause. 2005; 12:421–7.

5. Tsai E, Yang C, Chen H, Wu C, Lee J. Bladder neck circulation by Doppler ultrasonography in postmenopausal women with urinary stress incontinence. Obstet Gynecol. 2001; 98:52–6.

6. Zhu Q, Ritchie J, Marouf N, Dion SB, Resnick NM, Elbadawi A, et al. Role of ovarian hormones in the pathogenesis of impaired detrusor contractility: evidence in ovariectomized rodents. J Urol. 2001; 166:1136–41.

7. Aikawa K, Sugino T, Matsumoto S, Chichester P, Whitbeck C, Levin RM. The effect of ovariectomy and estradiol on rabbit bladder smooth muscle contraction and morphology. J Urol. 2003; 170:634–7.

8. Parekh MH, Chichester P, Lobel RW, Aikawa K, Levin RM. Effects of castration on female rabbit bladder physiology and morphology. Urology. 2004; 64:1048–51.

9. Fleischmann N, Christ G, Sclafani T, Melman A. The effect of ovariectomy and longterm estrogen replacement on bladder structure and function in the rat. J Urol. 2002; 168:1265–8.

10. Andersson KE. Advances in the pharmacological control of the bladder. Exp Physiol. 1999; 84:195–213.

11. Levin RM, Shofer FS, Wein AJ. Estrogen-induced alterations in the autonomic responses of the rabbit urinary bladder. J Pharmacol Exp Ther. 1980; 215:614–8.
12. Shapiro E. Effect of estrogens on the weight and muscarinic cholinergic receptor density of the rabbit bladder and urethra. J Urol. 1986; 135:1084–7.

13. Longhurst PA, Kauer J, Leggett RE, Levin RM. The influence of ovariectomy and estradiol replacement on urinary bladder function in rats. J Urol. 1992; 148:915–9.

14. Palea S, Angel I. The effect of ovariectomy on the contractile response of the rat isolated detrusor muscle and urethra. Life Sci. 1997; 61:PL21–6.

15. Susset JG, Servot-Viguier D, Lamy F, Madernas P, Black R. Collagen in 155 human bladders. Invest Urol. 1978; 16:204–6.
16. Lin AD, Mannikarottu A, Kogan BA, Whitbeck C, Chichester P, Leggett RE, et al. Estrogen induces angiogenesis of the female rabbit bladder. J Endocrinol. 2006; 190:241–6.

17. Blacher J, Dabire H, Promies JP, Safar ME, Stimpel M. Long-term cardiovascular effects of high "osteoprotective" dose levels of 17-beta estradiol in spontaneously hypertensive rats. Cardiovasc Drugs Ther. 2000; 14:303–7.
18. Juan YS, Mannikarottu A, Kogan BA, Leggett RE, Whitbeck C, Chichester P, et al. The effect of low-dose estrogen therapy on ovariectomized female rabbit bladder. Urology. 2008; 71:1209–13.

19. Longhurst PA, Uvelius B. Pharmacological techniques for the in vitro study of the urinary bladder. J Pharmacol Toxicol Methods. 2001; 45:91–108.

20. Wang P, Luthin GR, Ruggieri MR. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J Pharmacol Exp Ther. 1995; 273:959–66.
21. Longhurst PA, Leggett RE, Briscoe JA. Characterization of the functional muscarinic receptors in the rat urinary bladder. Br J Pharmacol. 1995; 116:2279–85.

22. Matsui M, Motomura D, Karasawa H, Fujikawa T, Jiang J, Komiya Y, et al. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci USA. 2000; 97:9579–84.

23. Stengel PW, Gomeza J, Wess J, Cohen ML. M2 and M4 receptor knockout mice: muscarinic receptor function in cardiac and smooth muscle in vitro. J Pharmacol Exp Ther. 2000; 292:877–85.
24. Matsumoto M, Watanabe T, Miyagawa I. Effects of longterm estradiol treatment on the contractile response to muscarine and muscarinic receptor subtypes in the bladder of aged female rats. Biomed Res. 2008; 28:309–14.

Go to : 
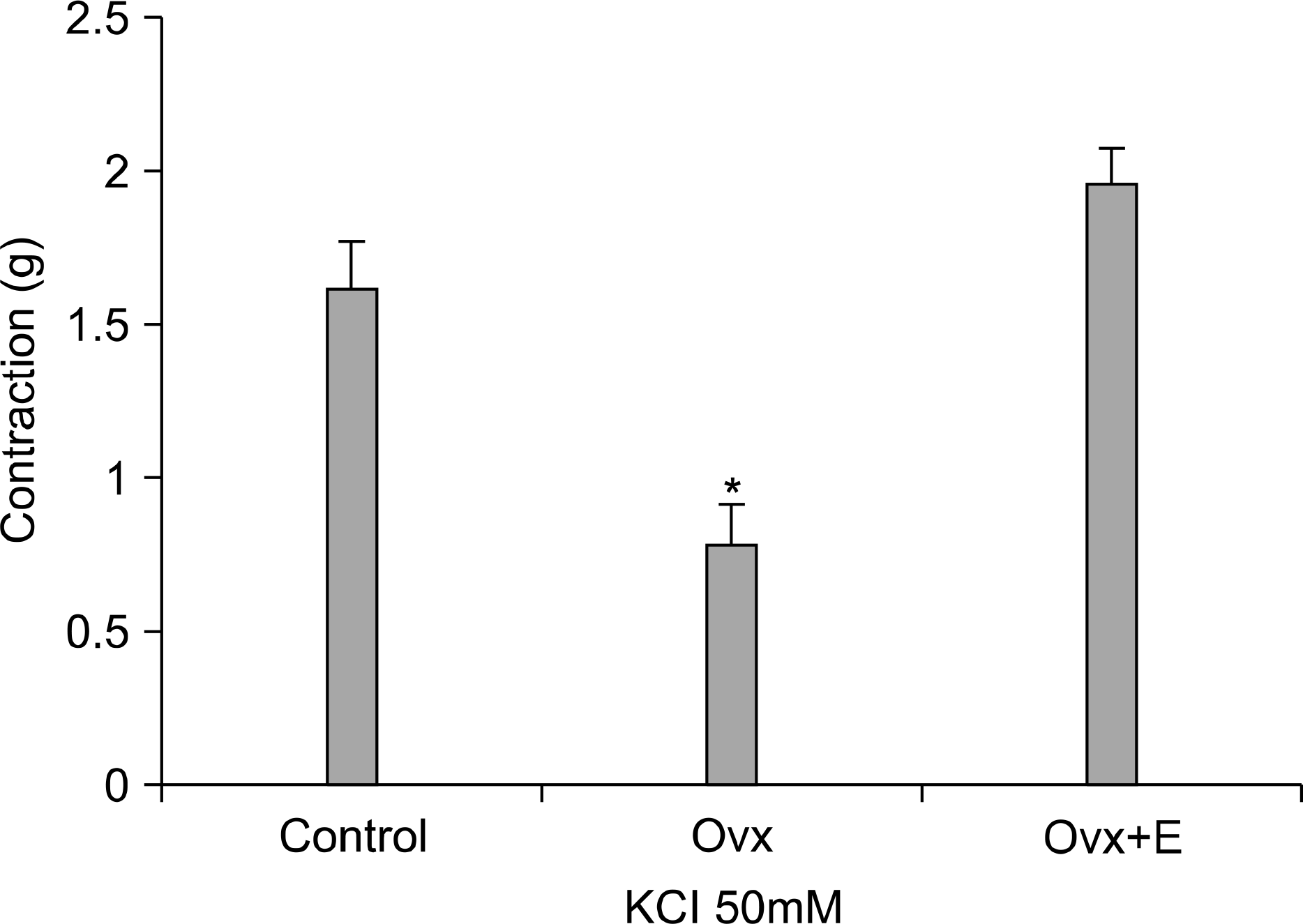 | Fig. 1.KCl 50mM induced contractile response of the rat bladder. The ovariectomized (Ovx) group showed a decreased contractile response to KCl 50mM compared with the control group and the Ovx+estradiol treated (Ovx+E) group. Each bar is the mean±SE. ∗: significantly different from the control group and the Ovx+E group (p<0.05). |
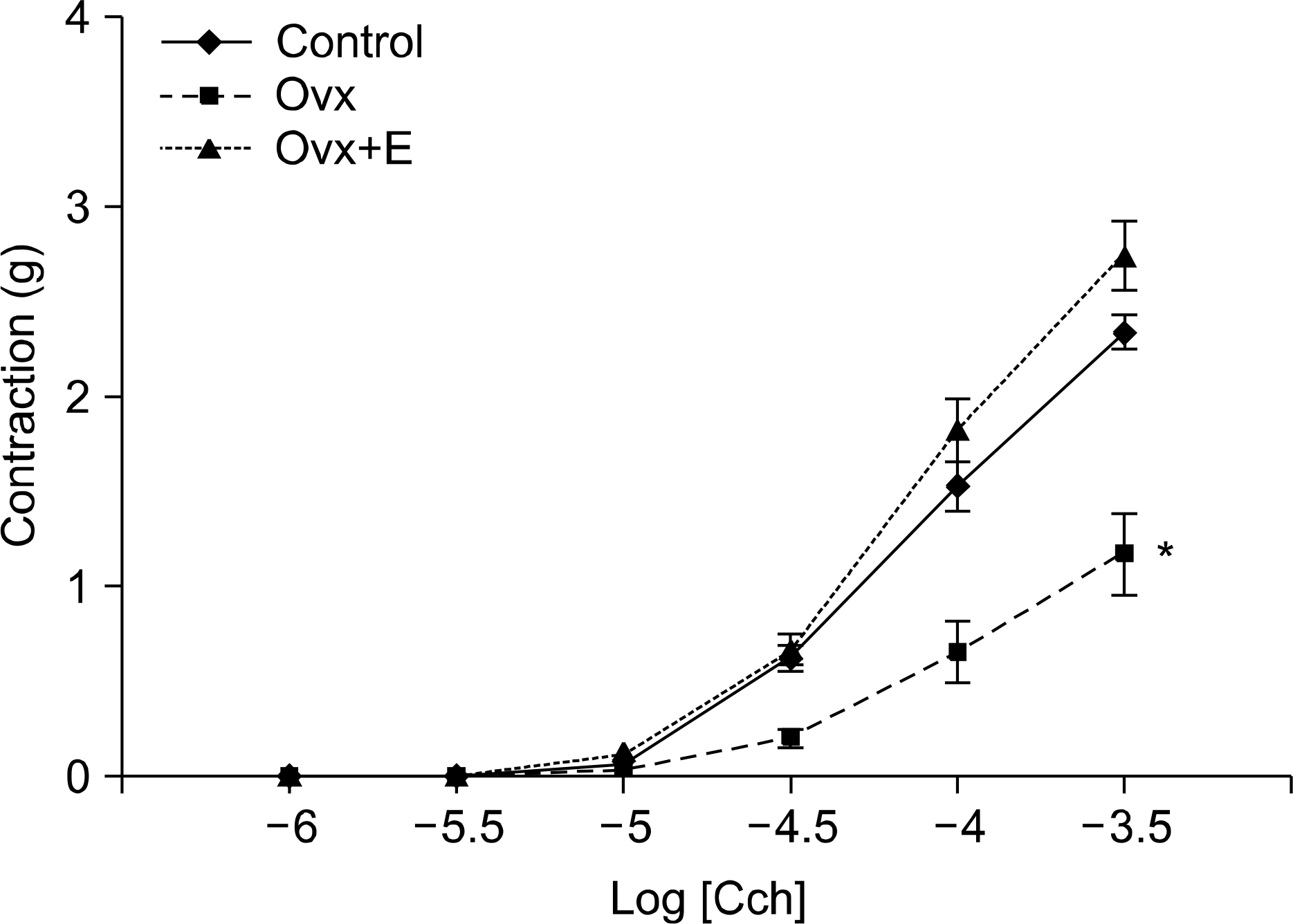 | Fig. 2.Carbachol (Cch) induced contractile responses of the rat bladder. The ovariectomized (Ovx) group showed decreased contractile responses to Cch, as compared with the control group and the Ovx+estradiol treated (Ovx+E) group. Values are plotted as means±SE. ∗: significantly different from the control group and the Ovx+E group (p<0.05). |
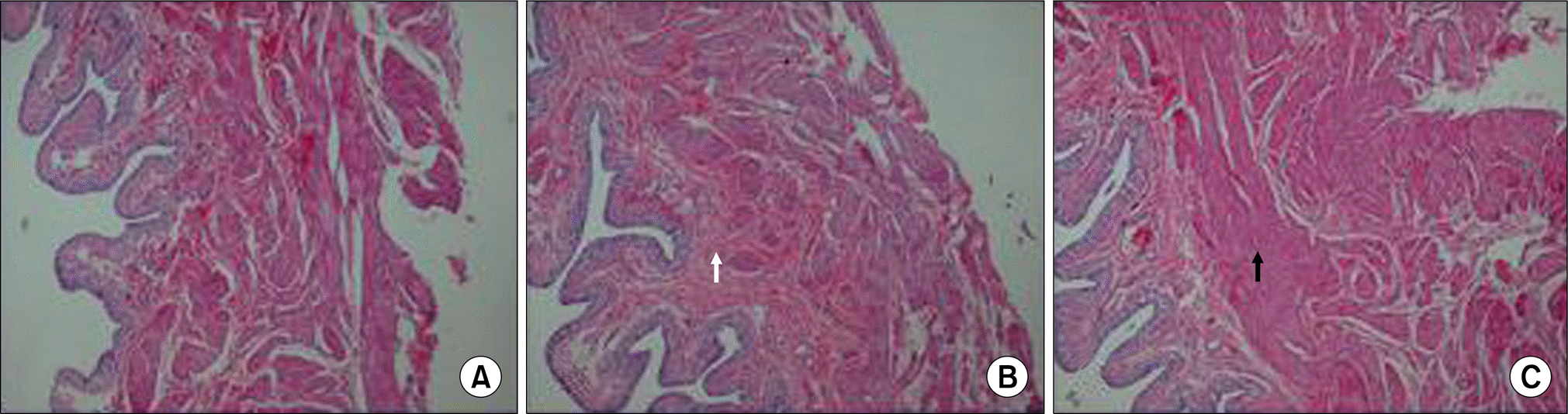 | Fig. 3.Representative photomicrographs showing staining of the control group (A), the Ovx group (B) and the Ovx+E group (C). The connective tissue in the Ovx group was markedly increased (white arrow) more than that in the control group and the Ovx+E group. Smooth muscle hypertrophy was observed in the Ovx+E group (black arrow) (H&E stain, original magnification, x40). |
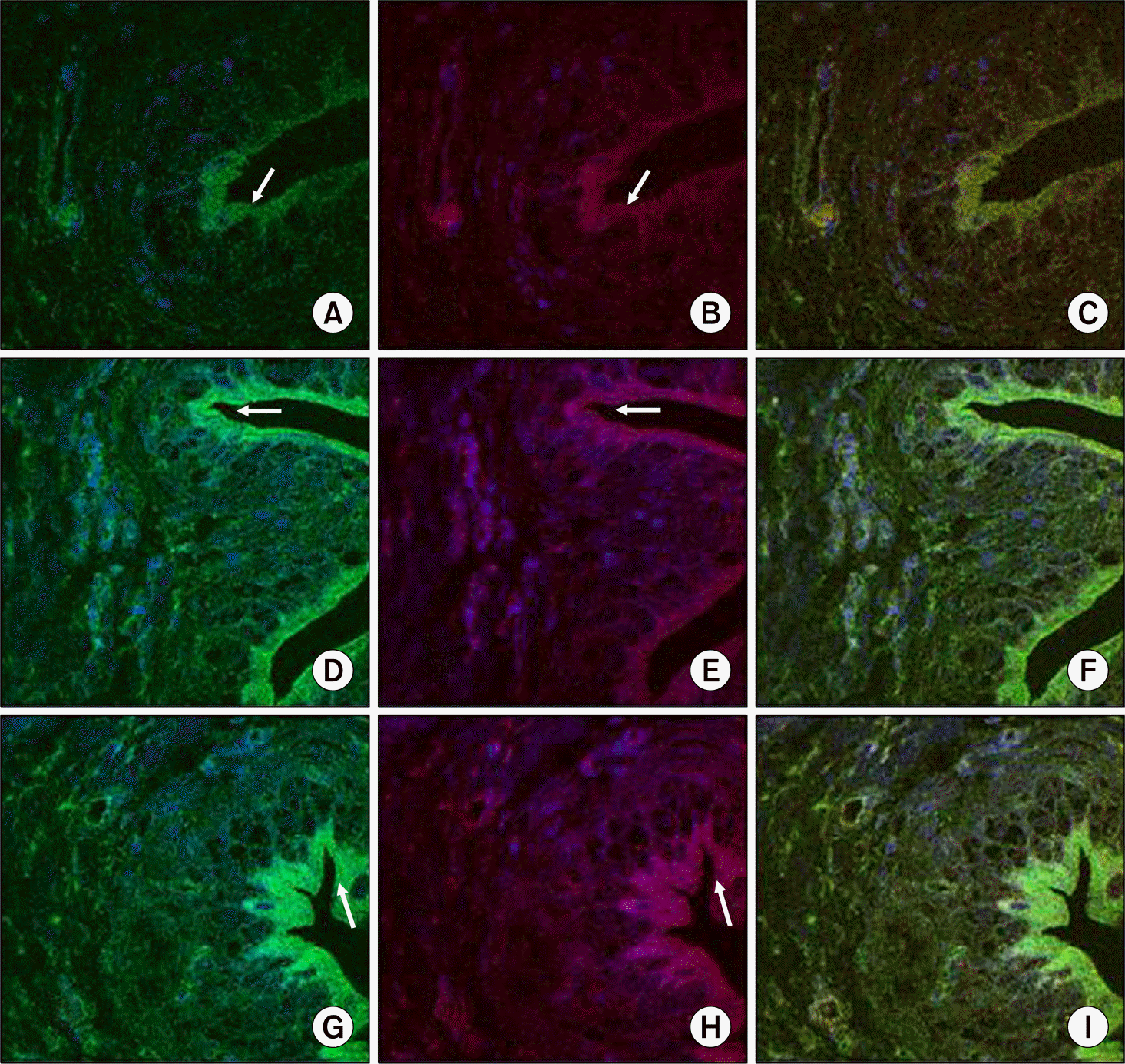 | Fig. 4.Comparisons of the M3 and M2 receptor expressions among the three groups on the immunofluorescent study. The expressions of M3 (green) and M2 (red) were showed in the control group (A, B), the Ovx group (D, E) and the Ovx+E group (G, H). Merged images of the M3 and M2 were showed in the control group (C), the Ovx group (F) and the Ovx+E group (I). The M3 receptor expressed in the epithelial area (arrow), and the M2 receptor had a similar pattern. The M3 receptor was more strongly expressed in the Ovx+E and Ovx groups than that in the control group. The M2 receptor was more strongly expressed in the Ovx+E group than that in the Ovx group and the control group (original magnification, x400). |
Table 1.
Comparisons of the M2 receptor expression among the three groups
| Group | Density of M2 receptor | |
|---|---|---|
| Epithelial layer | Muscle layer | |
| Control | 82.2±3.7 | 55.4±2.1 |
| Ovx | 94.2±4.1 | 56.2±1.5 |
| Ovx+E | 113.2±6.0∗† | 63.6±2.3∗† |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download