Abstract
Purpose
Acute or chronic prostatic inflammation exists to varying degrees in surgical specimens of prostates, extirpated for the treatment of benign prostatic hyperplasia (BPH). We investigated the relationship between acute urinary retention (AUR) and intraprostatic inflammation.
Materials and Methods
Between January 1997 and December 2006, 221 patients underwent transurethral resection of the prostate (TURP) for the treatment of BPH. The patients were divided into 2 groups based on the indication for surgery; an AUR group and a lower urinary tract symptoms (LUTS) group. The area of acute inflammation, the extent, and the aggressiveness of chronic inflammation were classified into four grades. The grades of inflammation, prostate volume, age, serum prostate-specific antigen (PSA), and prior medical treatment were compared between the two groups. All specimens were reviewed by one pathologist.
Results
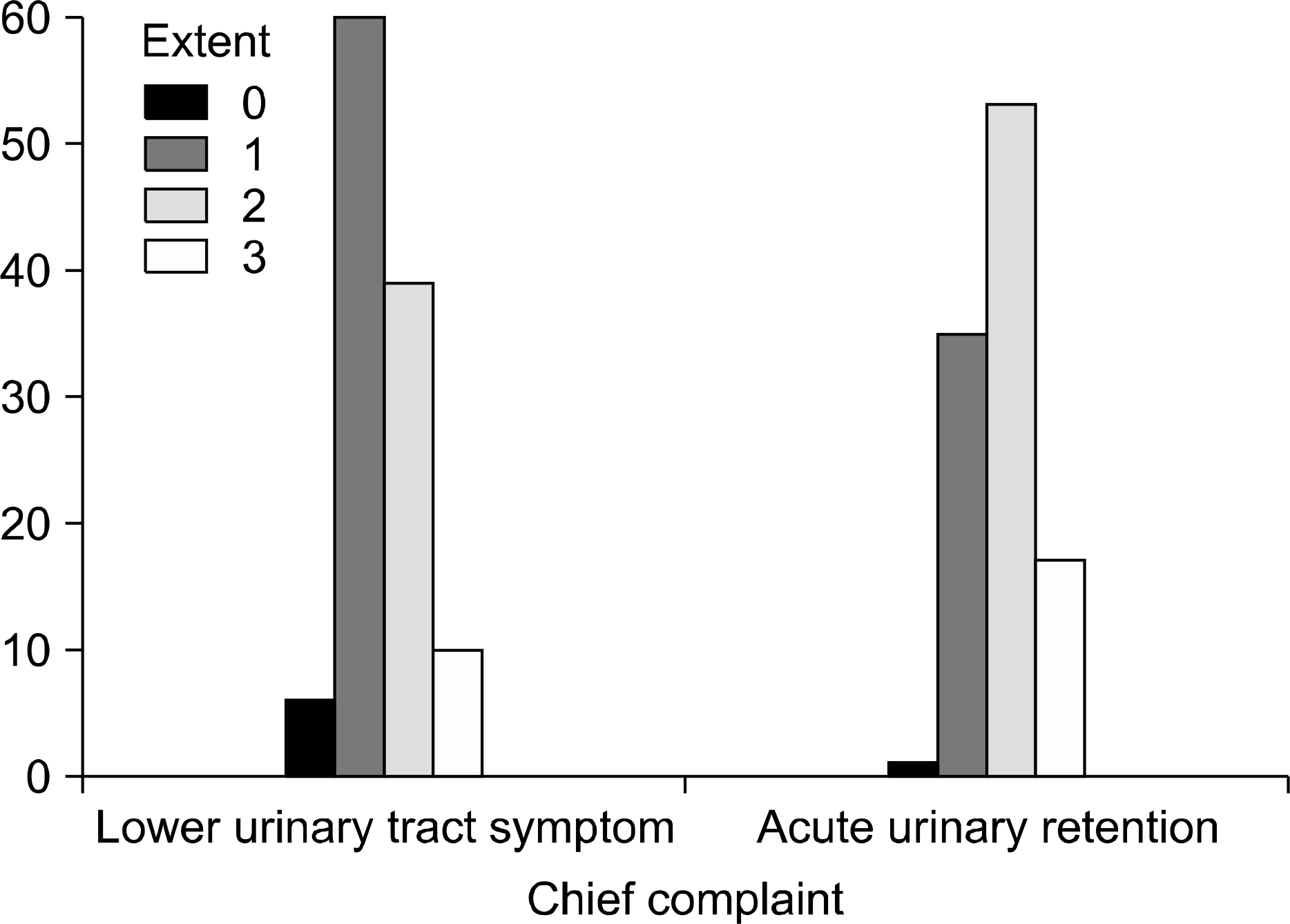
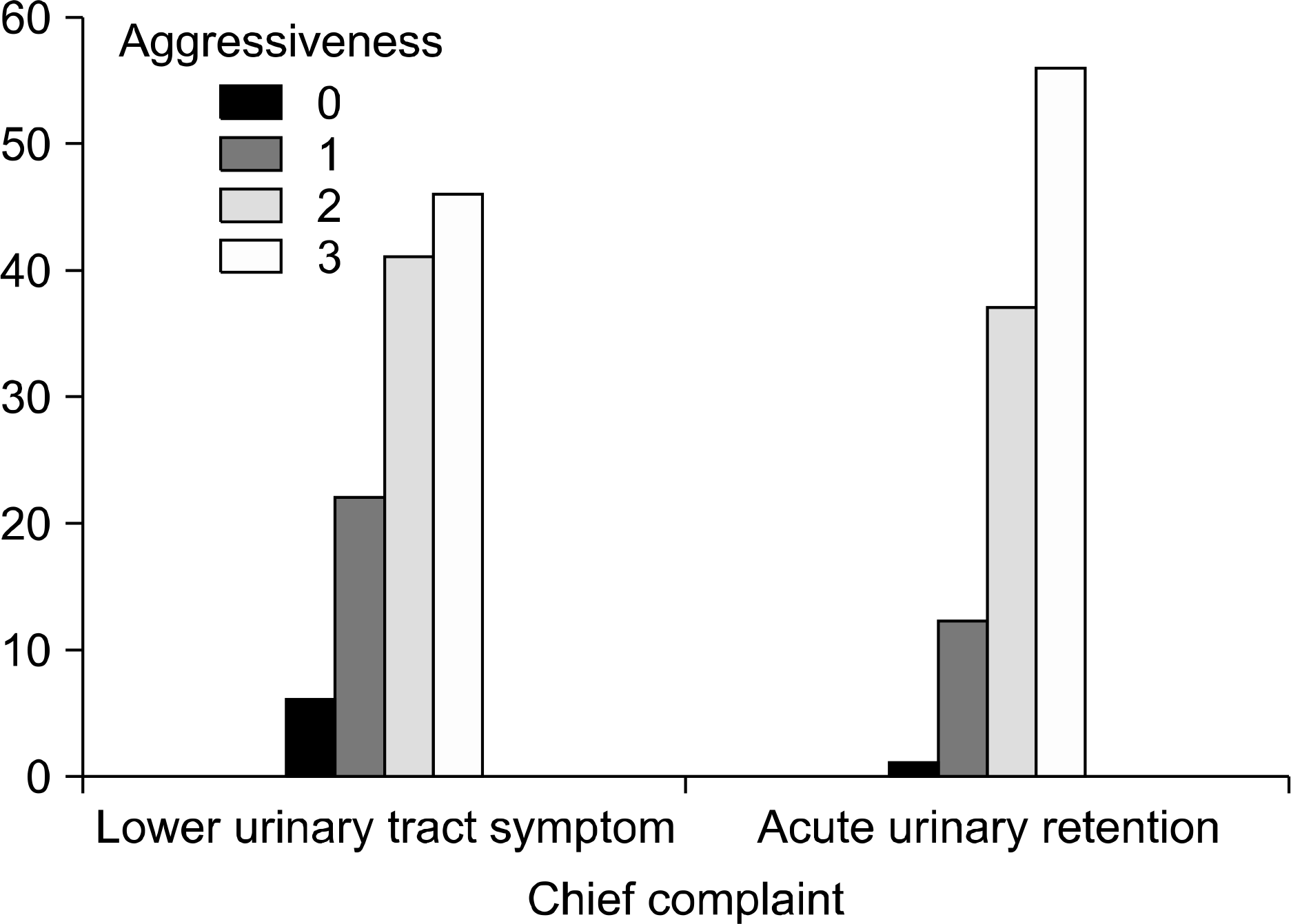
The AUR group consisted of 106 (47.9%) patients, and the LUTS group consisted of 115 (52.1%) patients. There were no statistical differences between the two groups with respect to the mean values of the age, prostate size, and severity of chronic inflammation. There was a significant relationship between AUR and the areas of acute inflammation, and the extent of chronic inflammation (p=0.014 and p=0.003, respectively). The aggressiveness of chronic inflammation had no relationship with AUR (p=0.062). The serum PSA level was higher in the AUR group than the LUTS group (11.5 vs. 5.3ng/ml, respectively).
Go to : 
References
1. Emberton M, Anson K. Acute urinary retention in men: an age old problem. BMJ. 1999; 318:921–5.
2. Shah T, Palit V, Biyani S, Elmasry Y, Puri R, Flannigan GM. Randomised, placebo controlled, double blind study of alfuzosin SR in patients undergoing trial without catheter following acute urinary retention. Eur Urol. 2002; 42:329–32.

3. Kefi A, Koseoglu H, Celebi I, Yorukoglu K, Esen A. Relation between acute urinary retention, chronic prostatic inflammation and accompanying elevated prostate-specific antigen. Scand J Urol Nephrol. 2006; 40:155–60.

4. Tuncel A, Uzun B, Eruyar T, Karabulut E, Seckin S, Atan A. Do prostatic infarction, prostatic inflammation and prostate morphology play a role in acute urinary retention? Eur Urol. 2005; 48:277–83.

5. Mishra VC, Allen DJ, Nicolaou C, Sharif H, Hudd C, Karim OM, et al. Does intraprostatic inflammation have a role in the pathogenesis and progression of benign prostatic hyperplasia? BJU Int. 2007; 100:327–31.

6. Kim MJ, Lee JG, Cheon J. The factors that influence the success rate of treatment without using a catheter for the management of acute urinary retention: comparison of in-and-out catheterization and Foley indwelling catheterization. Korean J Urol. 2008; 49:337–42.

7. Chang HS, Park CH, Kim CI. Transitional zone volume: a predictor of acute urinary retention in patients with benign prostatic hyperplasia. Korean J Urol. 2005; 46:259–63.
8. Lee SJ, Kim YT, Lee TY, Woo YN. Analysis of risk factors for acute urinary retention after non-urogenital surgery. Korean J Urol. 2007; 48:1277–84.

9. Irani J, Levillain P, Goujon JM, Bon D, Dore B, Aubert J. Inflammation in benign prostatic hyperplasia: correlation with prostate specific antigen value. J Urol. 1997; 157:1301–3.

10. Delongchamps NB, de la Roza G, Chandan V, Jones R, Sunheimer R, Threatte G, et al. Evaluation of prostatitis in autopsied prostates – Is chronic inflammation more associated with benign prostatic hyperplasia or cancer? J Urol. 2008; 179:1736–40.
11. Jacobsen SJ, Jacobson DJ, Girman CJ, Roberts RO, Rhodes T, Guess HA, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol. 1997; 158:481–7.

12. Kurita Y, Masuda H, Terada H, Suzuki K, Fujita K. Transition zone index as a risk factor for acute urinary retention in benign prostatic hyperplasia. Urology. 1998; 51:595–600.

13. Kim JH, Kim HG, Park WH. The predictors of acute urinary retention in patients with benign prostatic hyperplasia. Korean J Urol. 2002; 43:949–55.
14. Fowler FJ Jr, Wennberg JE, Timothy RP, Barry MJ, Mulley AG Jr, Hanley D. Symptom status and quality of life following prostatectomy. JAMA. 1988; 259:3018–22.

15. Mebust WK, Holtgrewe HL, Cockett AT, Peters PC. Transurethral prostatectomy: immediate and postoperative complications. A cooperative study of 13 participating institutions evaluating 3,885 patients. J Urol. 1989; 141:243–7.
16. Roehrborn CG, McConnell JD, Lieber M, Kaplan S, Geller J, Malek GH, et al. Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. Urology. 1999; 53:473–80.

17. Kim CI, Chang HS, Kim BK, Park CH. Long-term results of medical treatment in benign prostatic hyperplasia. Urology. 2006; 68:1015–9.

18. Lynch TH. Doxazosin and finasteride alone or in combination: the PREDICT study. BJU Int. 2003; 91:591–2.

19. McConnell JD, Roehrborn CG, Bautista OM, Andriole GL Jr, Dixon CM, Kusek JW, et al. The longterm effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003; 349:2387–98.

20. Di Silverio F, Gentile V, De Matteis A, Mariotti G, Giuseppe V, Luigi PA, et al. Distribution of inflammation, pre-malignant lesions, incidental carcinoma in histologically confirmed benign prostatic hyperplasia: a retrospective analysis. Eur Urol. 2003; 43:164–75.

21. Roberts RO, Lieber MM, Rhodes T, Girman CJ, Bostwick DG, Jacobsen SJ. Prevalence of a physician-assigned diagnosis of prostatitis: the Olmsted County Study of Urinary Symptoms and Health Status Among Men. Urology. 1998; 51:578–84.

22. Roberts RO, Jacobson DJ, Girman CJ, Rhodes T, Lieber MM, Jacobsen SJ. Prevalence of prostatitis-like symptoms in a community based cohort of older men. J Urol. 2002; 168:2467–71.

23. Roehrborn CG, Kaplan SA, Noble WD, Slawin KM, McVary KT, Kusek JW. The impact of acute or chronic inflammation in baseline biopsy on the risk of clinical progression of BPH. Results from the MTOPS study. J Urol. 2005; 173(Suppl):346. abstract 1277.
24. Isaacs JT, Coffey DS. Etiology and disease process of benign prostatic hyperplasia. Prostate. 1989; 2(Suppl):33–50.

25. Nickel JC, Downey J, Young I, Boag S. Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int. 1999; 84:976–81.

26. Collins MM, Meigs JB, Barry MJ, Walker Corkery E, Giovannucci E, Kawachi I. Prevalence and correlates of prostatitis in the health professionals follow-up study cohort. J Urol. 2002; 167:1363–6.

27. Kramer G, Marberger M. Could inflammation be a key component in the progression of benign prostatic hyperplasia? Curr Opin Urol. 2006; 16:25–9.

Go to : 
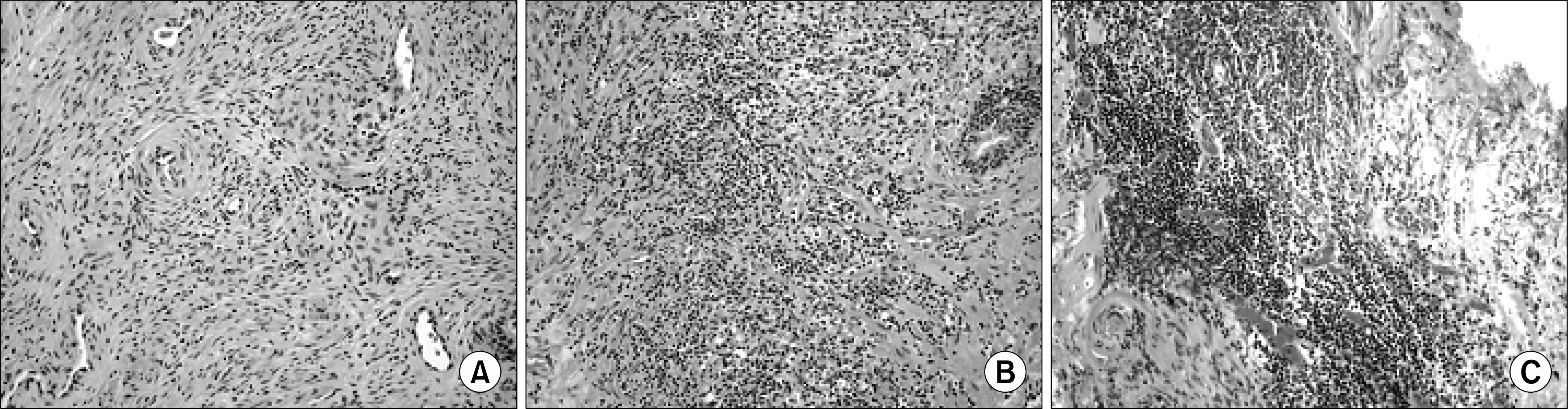 | Fig. 1.Extent of chronic inflammation was graded on a 4-point scale. (A) Grade 1: scattered inflammatory cell infiltrate within the stroma without lymphoid nodules (H&E, x200). (B) Grade 2: nonconfluent lymphoid nodules (H&E, x200). (C) Grade 3: large inflammatory areas with confluence of infiltrate (H&E, x200). |
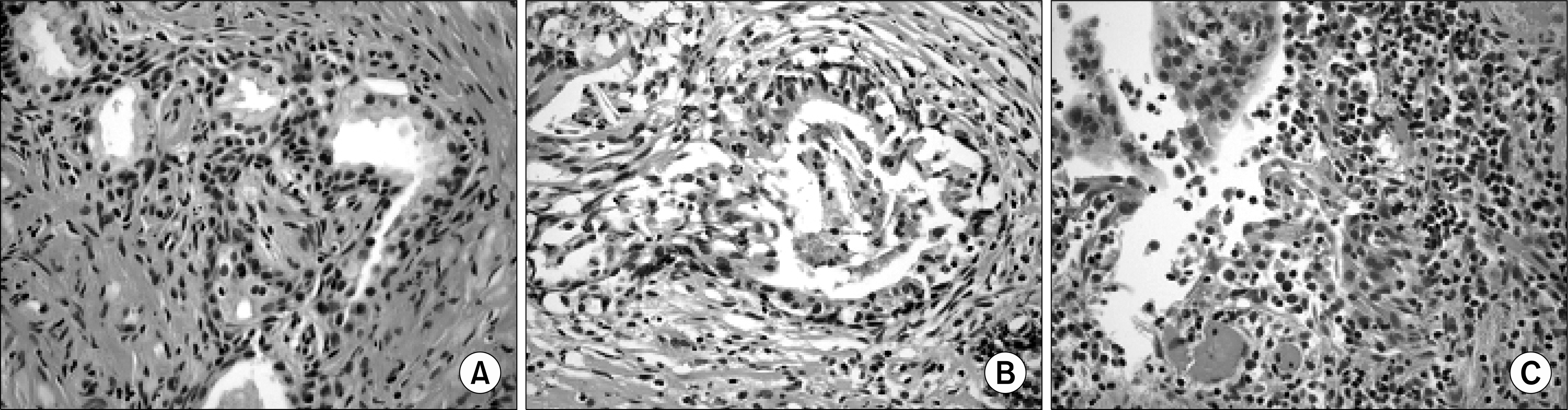 | Fig. 2.Aggressiveness of chronic inflammation was graded on a 4-point scale. (A) Grade 1: contact between inflammatory cell infiltrate and glandular epithelium (however, minimal epithelium dissociation can be observed without disruption) (H&E, x400). (B) Grade 2: interstitial inflammatory infiltrate associated with a clear but limited (<25% of the examined material) glandular epithelium disruption (H&E, x400). (C) Grade 3: glandular epithelium disruption on >25% of the examined material (H&E, x400). |
Table 1.
Characteristics of the patients in the AUR and LUTS groups
Table 2.
Analysis for histologic inflammation in the AUR and LUTS groups




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download