Abstract
Purpose
There are little research about the factors of DNA damage and repair that might cause renal parenchymal damage under the condition of hydronephrosis. In this study, we studied the expressions of p53, γ-H2AX and Ku70/Ku80 in rat kidney under the condition of hydronephrosis.
Materials and Methods
16 Sprague-Dawley rats that were 7~8 weeks old were used. Partial ureteral obstruction was induced in 12 rats. And for the other 4 rats, a sham-operation was done as a control. The hydronephrosis-induced rats had their right kidney removed after 1, 2 and 3 weeks and the sham-operated rats underwent nephrectomy after 3 weeks. Those removed tissues were examined with immunohistochemical staining and western blot to confirm the degree of expression of p53, γ-H2AX and Ku70/Ku80.
Results
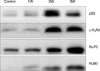
The expressions of p53, γ-H2AX and Ku70/Ku80 caused by hydronephrosis in the rat increased as time passed, and these expressions of controls were in a low level or they were negative. These results were similar with the results of the immunohistochemical staining and Western blot.
Conclusions
This study confirmed that the protein expressions of γ-H2AX, p53, and Ku70/Ku80 are increased, and these expressions are the DNA damage-related factors in the renal parenchyma of hydronephrosisinduced rats. We confirmed the possibility that DNA double strand breaks (DSBs) might be the main mechanism that induces renal parenchymal damage under the condition of hydronephrosis.
Figures and Tables
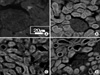 | Fig. 1Immunohistochemical staining for p53 in hydronephrosis-induced rats. The expressions of p53 after 1, 2 and 3 weeks (B, C and D) were increased compared to the control group (A), and these expressions increased with time. The expression of the tubules was higher than that of the glomerulus, and tubule expression increased with time. (A) Right kidney of the sham-operated rats after 3 weeks (control group), (B) right kidney of the hydronephrosis-induced rats after 1 week, (C) right kidney of the hydronephrosis-induced rats after 2 weeks, (D) right kidney of the hydronephrosis-induced rats after 3 weeks. |
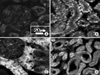 | Fig. 2Immunohistochemical staining for γ-H2AX in the hydronephrosis-induced rats. The expressions of γ-H2AX after 1, 2 and 3 weeks (B, C and D) were increased compared to the control group (A), and these expressions increased as time passed. The expression of γ-H2AX in the tubule was higher than that of glomerulus. (A) Right kidney of the shamoperated rats after 3 weeks (control group), (B) right kidney of the hydronephrosis-induced rats after 1 week, (C) right kidney of the hydronephrosis-induced rats after 2 weeks, (D) right kidney of the hydronephrosis-induced rats after 3 weeks. |
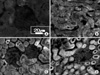 | Fig. 3Immunohistochemical staining for Ku70 in hydronephrosis-induced rats. The expressions of Ku70 after 1, 2 and 3 weeks (B, C and D) were increased compared to the control group (A), and these expressions increased as time passed. The expression of Ku70 in the tubule was higher than that of the glomerulus. (A) Right kidney of the sham-operated rats after 3 weeks (control group), (B) right kidney of the hydronephrosis-induced rats after 1 week, (C) right kidney of the hydronephrosis-induced rats after 2 weeks, (D) right kidney of the hydronephrosis-induced rats after 3 weeks. |
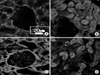 | Fig. 4Immunohistochemical staining for Ku80 in hydronephrosis-induced rats. The expressions of Ku80 after 1, 2 and 3 weeks (B, C and D) were increased compared to the control group (A), and these expressions increased as time passed. The expression of Ku80 in the tubule was higher than that of glomerulus. (A) Right kidney of the sham-operated rats after 3 weeks (control group), (B) right kidney of the hydronephrosis-induced rats after 1 week, (C) right kidney of the hydronephrosis-induced rats after 2 weeks, (D) right kidney of the hydronephrosis-induced rats after 3 weeks. |
References
1. Gobe GC, Axelsen RA. Genesis of renal tubular atrophy in experimental hydronephrosis in the rat. Role of apoptosis. Lab Invest. 1987. 56:273–281.
2. Truong LD, Sheikh-Hamad D, Chakraborty S, Suki WN. Cell apoptosis and proliferation in obstructive uropathy. Semin Nephrol. 1998. 18:641–651.
3. Choi YJ, Baranowska-Daca E, Nguyen V, Koji T, Ballantyne CM, Sheikh-Hamad D, et al. Mechanism of chronic obstructive uropathy: increased expression of apoptosis-promoting molecules. Kidney Int. 2000. 58:1481–1491.
4. Truong LD, Petrusevska G, Yang G, Gurpinar T, Shappell S, Lechago J, et al. Cell apoptosis and proliferation in experimental chronic obstructive uropathy. Kidney Int. 1996. 50:200–207.
5. Truong LD, Choi YJ, Tsao CC, Ayala G, Sheikh-Hamad D, Nassar G, et al. Renal cell apoptosis in chronic obstructive uropathy: the roles of caspases. Kidney Int. 2001. 60:924–934.
6. Kim HG, Paick SH, Lho YS, Kim HH, Kwak C. The protein expressions of apoptosis-associated genes in the obstructed ureters of rats. Korean J Urol. 2006. 47:189–194.
7. Fisher DE. The p53 tumor suppressor: critical regulator of life and death in cancer. Apoptosis. 2001. 6:7–15.
8. Lugar K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature. 1997. 389:251–260.
9. Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998. 273:5858–5868.
10. Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999. 146:905–916.
11. Tuteja R, Tuteja N. Ku autoantigen: a multifunctional DNA-binding protein. Crit Rev Biochem Mol Biol. 2000. 35:1–33.
12. Eun JU, Kim TK. Renal hemodynamic changes in acute ureteral obstruction. Korean J Urol. 1995. 36:932–938.
13. Brkljacic B, Drinkovic I, Sabljar-Matovinovic M, Soldo D, Morovic-Vergles J, Vidjak V, et al. Intrarenal duplex Doppler sonographic evaluation of unilateral native kidney obstruction. J Ultrasound Med. 1994. 13:197–204.
14. Platt JF. Duplex Doppler evaluation of native kidney dysfunction: obstructive and nonobstructive disease. AJR Am J Roentgenol. 1992. 158:1035–1042.
15. Singer B, Kusmierek JT. Chemical mutagenesis. Annu Rev Biochem. 1982. 51:655–693.
16. Bridges BA. DNA repair: polymerases for passing lesions. Curr Biol. 1999. 9:R475–R477.
17. Citterio E, Vermeulen W, Hoeijmakers JH. Transcriptional healing. Cell. 2000. 101:447–450.
18. Lane DP. Cancer. p53, guardian of the genome. Nature. 1992. 358:15–16.
19. Scheinman M, Ascher E, Kallakuri S, Hingorani A, Gade P, Sherman M, et al. p53 gene transfer to the injured rat carotid artery promotes apoptosis. Surgery. 1999. 126:863–868.
20. Darnton SJ. Demystified... p53. Mol Pathol. 1998. 51:248–253.
21. Wu RS, Panusz HT, Hatch CL, Bonner WM. Histones and their modifications. CRC Crit Rev Biochem. 1986. 20:201–263.
22. Rogakou EP, Nieves-Neira W, Boon C, Pommier Y, Bonner WM. Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J Biol Chem. 2000. 275:9390–9395.
23. Sedelnikova OA, Rogakou EP, Panyutin IG, Bonner WM. Quantitative detection of (125)IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Radiat Res. 2002. 158:486–492.
24. Rathmell WK, Chu G. Involvement of the Ku autoantigen in the cellular response to DNA double-strand breaks. Proc Natl Acad Sci USA. 1994. 91:7623–7627.
25. Walker JR, Corpina RA, Goldberg J. Structure of the Ku heterodimer bound to DNA and its implications for doublestrand break repair. Nature. 2001. 412:607–614.
26. Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001. 27:247–254.
27. Feldmann E, Schmiemann V, Goedecke W, Reichenberger S, Pfeiffer P. DNA double-strand break repair in cell-free extracts from Ku80-deficient cells: implications for Ku serving as an alignment factor in non-homologous DNA end joining. Nucleic Acids Res. 2000. 28:2585–2596.
28. Gullo C, Au M, Feng G, Teoh G. The biology of Ku and its potential oncogenic role in cancer. Biochim Biophys Acta. 2006. 1765:223–234.
29. Li GC, Ouyang H, Li X, Nagasawa H, Little JB, Chen DJ, et al. Ku70: a candidate tumor suppressor gene for murine T cell lymphoma. Mol Cell. 1998. 2:1–8.
30. Gu Y, Seidl KJ, Rathbun GA, Zhu C, Manis JP, van der Stoep N, et al. Growth retardation and leaky SCID phenotype of Ku70-deficient mice. Immunity. 1997. 7:653–665.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download