The Incidence of port site metastases after laparoscopic surgery in urologic oncology has been estimated at 0.09% to 0.73%.1,2 Port site metastasis following laparoscopic radical nephrectomy is very rare. Many studies have suggested the pathogenesis, risk factors, preventive measures for port site metastases of renal cell carcinoma after laparoscopic radical nephrectomy.3,4 We report a case of local recurrence with port site metastasis which was incidentally found after laparoscopic radical nephrectomy at that time we stood in the early part of the learning curve for laparoscopic surgery.
CASE REPORT
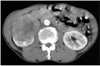
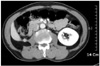
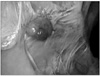
A 62-year old man presented with 5 kg weight loss for 6 months without flank pain, hematuria and abdominal palpable mass. Abdominal computed tomography scan with intravenous contrast imaging demonstrated an approximately 8.3×6.7×6.1 cm sized solid mass in the anterior portion of the right kidney (Fig. 1). Serum hemoglobin levels were 11.4 mg/dl in complete blood count. Other serologic laboratory findings were within normal range for his age. The mass lesion was suspicious for renal cell carcinoma, but evidence of distant metastases was not found. The patient underwent laparoscopic radical nephrectomy by transperitoneal route. An 12 mm trocar was inserted in the right lateral border of rectus muscle 2 cm below the umbilicus and was attached the camera to monitor the insertion of the subsequent trocars. A 10 mm trocar was inserted in the midclavicular line 2 cm below the costal margin. A 5 mm trocar was placed in the anterior axillary line at the tip of the 12th rib and a 5 mm trocar was inserted in the same line 1 cm below the umbilicus. Through 10 cm extended incision of the 12 mm port, intact surgical specimen was extracted without using a retrieval bag. The final pathologic finding of renal mass was consistent with clear cell type renal cell carcinoma. Fuhrman nuclear grade and the final pathologic stage were the grade III and pT2N0M0, respectively. Cancer cell was not detected in resected margin of surgical specimen. Twenty months after the nephrectomy for primary tumor, abdominal computed tomography scan imaging demonstrated a small solid mass in retroperitoneum. The size of the mass increased on abdominal computed tomography scan imaging at additional 4 months later (Fig. 2). The mass was expected as local recurrence of renal cell carcinoma. We tried laparoscopic mass resection and pathologic confirmation of the recurred mass. We incidentally found a 1×1 cm sized solid mass under the 4th port site scar of the past laparoscopic radical nephrectomy during laparoscopic examination. The mass was not detected on abdominal computed tomography scan imaging (Fig. 3). The mass was enveloped in peritoneum and fixed on the inner abdominal wall. Retroperitoneal mass, which was detected by computed tomography and was found incidentally was completely resected by laparoscopic surgical procedures. The final pathologic finding of two resected tumors was consistent with renal cell carcinoma and Fuhrman nuclear grade III (Fig. 4). Also microscopic finding of the incidentally found mass specimen presented tumor with suture granuloma. We diagnosed him as port site metastasis with local recurrence of renal cell carcinoma. Combination immunotherapy (Interferone-alpha+IL-2) was administered to the patient with his acceptance of the adjuvant therapy. Four months after the combination immunotherapy, computed tomography and other studies for metastasis found no evidence of local recurrence and distant metastases.
DISCUSSION
Port site metastases following laparoscopic surgery are early recurrent tumorous lesions. The lesions develop locally in the abdominal wall within the scar tissue of one or more trocar sites of previous laparoscopic surgery.5 Port site metastases are not associated with diffuse peritoneal carcinomatosis.6 Incidence of port site metastases after laparoscopic surgery in urologic oncology was very low in the two large surveys, and port site metastases of renal cell carcinoma were not reported in these surveys.1,2 First case of a port site metastasis after laparoscopic oncologic surgery in urologic field was reported at 1994.7 In the case, laparoscopic pelvic lymphadenectomy previously was done. For the first time, Fentie DD et al.8 reported port site metastasis of renal cell carcinoma after laparoscopic radical nephrectomy at 2000. Only additional two cases of port site metastasis were reported for port site metastases of renal cell carcinoma to our knowledge. The pathogenesis of port site metastases is not yet known but appears to be multifactorial. The postulated multiple factors for port site metastases are natural tumor behavior, local wound factor, immune and stress response, and laparoscopic factors.1,9 In our case, the primary high grade tumor and incomplete skill of surgeon may have contributed to cancer implantation in the previous port site. Laparoscopic surgical techniques are important in port site metastases. Standardized and meticulous surgical technique reduce the incidence of port site metastases.5 In our patient, primary tumor specimen was extracted without using a retrieval bag, abdominal wall recurrence was under the 4th port site scar. Cancer cells might be spread due to direct specimen manipulation without using retrieval bag. Laparoscopic instruments and port sites might be directly contaminated by cancer cells during laparoscopic manipulation. In our case, another possible explanation for development of port site metastasis is exfoliation and aerosolization of cancer cells. Exfoliated and aerosolized cancer cells might be indirectly migrated to abdominal wall of the port site by gas leakage around the trocar. Specific protective measures for port site metastses in laparoscopic surgery can reduce the incidence of port site metastases.5 We did little techniques for prevention of port site metastases such as trocar fixation, prevention of gas leak and disinfection of instruments with povidone-iodine solution, although the primary tumor was expected to potentially aggressive tumor. Port site metastases following laparoscopic radical nephrectomy are very rare but laparoscopic surgeon have to keep oncologic principles strictly and consider potential preventive techniques for prevention of port site metastases when doing laparoscopic radical nephrectomy for renal cell carcinoma.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download