Abstract
Purpose
Chronic bacterial prostatitis (CBP) is one of the most common urological diseases in adult males, for which antibiotic therapy is the gold standard treatment. However, long-term therapy has many side effects and results in antibiotic resistance. For these reasons, a new treatment modality, which can replace the traditional antibiotic therapy, is required. Ginse-noside has various confirmed bacterial clearance effects in the respiratory system and on immune modulation. UrovaxomⓇ also has confirmed effects on immune modulation, with a preventive effect on recurrent urinary tract infection (UTI). Therefore, the anti-bacterial and synergistic effects of gin-senoside and UrovaxomⓇ were evaluated for the treatment of CBP in ana-nimal model.
Materials and Methods
The 60 rats demonstrating CBP were randomly divided into 6 groups; the control, ciprofloxacin, ginsenoside, UrovaxomⓇ, ciprofloxacin with ginsenoside, and ciprofloxacin with UrovaxomⓇ groups. All drug treatments were conducted for 2 weeks. After treatment, the results were analysed microbiologically and histopathologically.
Results
The microbiological cultures of the prostate and urine samples and the histological findings of the prostate demonstrated reduced bacterial growth and improved inflammatory responses in all the experimental groups compared with the control group. The ciprofloxacin, and UrovaxomⓇ with ciprofloxacin groups showed statistically significant decreases in bacterial growth and improvements in inflammatory responses compared to the control group (p<0.05). The ginsenoside with ciprofloxacin group showed no statistical significance (p>0.05), but the mean difference was very large.
Go to : 
References
1. Pfau A. Prostatitis. A continuing enigma. Urol Clin North Am. 1986; 13:695–715.
2. Nickel JC, Olson ME, Costerton JW. Rat model of experimental bacterial prostatitis. Infection. 1991; 19(Suppl 3):S126–30.

4. Song ZJ, Johansen HK, Faber V, Hoiby N. Ginseng treatment enhances bacterial clearance and decreases lung pathology in athymic rats with chronic P. aeruginosa pneumonia. APMIS. 1997; 105:438–44.
5. Ahn JY, Song JY, Yun YS, Jeong G, Choi IS. Protection of Staphylococcus aureus-infected septic mice by suppression of early acute inflammation and enhanced antimicrobial activity by ginsan. FEMS Immunol Med Microbiol. 2006; 46:187–97.
6. Park EK, Choo MK, Han MJ, Kim DH. Ginsenoside Rh1 possesses antiallergic and antiinflammatory activities. Int Arch Allergy Immunol. 2004; 133:113–20.

7. Matsuda H, Samukawa K, Kubo M. Anti-inflammatory activity of ginsenoside ro 1. Planta Med. 1990; 56:19–23.
8. Yu SC, Li XY. Effect of ginsenoside on IL-1 beta and IL-6 mRNA expression in hippocampal neurons in chronic inflammation model of aged rats. Acta Pharmacol Sin. 2000; 21:915–8.
9. Bauer HW, Rahlfs VW, Lauener PA, Blessmann GS. Prevention of recurrent urinary tract infections with immunoactive E. coli fractions: a metaanalysis of five placebocontrolled double-blind studies. Int J Antimicrob Agents. 2002; 19:451–6.

10. Lee SJ, Kim SW, Cho YH, Yoon MS. Anti-inflammatory effect of an Escherichia coli extract in a mouse model of lipopolysaccharide-induced cystitis. World J Urol. 2006; 24:33–8.

11. Naber KG, Sorgel F, Kees F, Jaehde U, Schumacher H. Pharmacokinetics of ciprofloxacin in young (healthy volunteers) and elderly patients, and concentrations in prostatic fluid, seminal fluid, and prostatic adenoma tissue following intravenous administration. Am J Med. 1989; 87:57S–9.
12. Seo SI, Lee SJ, Kim JC, Choi YJ, Kim SW, Hwang TK, et al. Effects of androgen deprivation on chronic bacterial prostatitis in a rat model. Int J Urol. 2003; 10:485–91.

14. Nickel JC, Olson ME, Barabas A, Benediktsson H, Dasgupta MK, Costerton JW. Pathogenesis of chronic bacterial prostatitis in an animal model. Br J Urol. 1990; 66:47–54.

15. Cho YH, Lee SJ, Lee JY, Kim SW, Lee CB, Lee WY, et al. Antibacterial effect of intraprostatic zinc injection in a rat model of chronic bacterial prostatitis. Int J Antimicrob Agents. 2002; 19:576–82.

16. Jantos C, Altmannsberger M, Weidner W, Schiefer HG. Acute and chronic bacterial prostatitis due to E-coli. Description of an animal model. Urol Res. 1990; 18:207–11.
17. Moon TD. Questionnaire survey of urologist and primary care physicians' diagnostic and treatment practices for prostatitis. Urology. 1997; 50:543–7.
18. Terai A, Yamamoto S, Mitsumori K, Okada Y, Kurazono H, Takeda Y, et al. Escherichia coli virulence factors and serotypes in acute bacterial prostatitis. Int J Urol. 1997; 4:289–94.

19. Neal DE Jr, Moon TD. Use of terazosin in prostatodynia and validation of a symptom score questionnaire. Urology. 1994; 43:460–5.

20. Winningham DG, Nemoy NJ, Stamey TA. Diffusion of antibiotics from plasma into prostatic fluid. Nature. 1968; 219:139–43.

21. Stamey TA, Meares EM Jr, Winningham DG. Chronic bacterial prostatitis and diffusion of dugs into prostatic fluid. J Urol. 1970; 103:187–94.
22. Xie X, Eberding A, Madera C, Fazli L, Jia W, Goldenberg L, et al. Rh2 synergistically enhances paclitaxel or mitoxantrone in prostate cancer models. J Urol. 2006; 175:1926–31.

23. Kim HS, Lee EH, Ko SR, Choi KJ, Park JH, Im DS. Effects of ginsenosides Rg3 and Rh2 on the proliferation of prostate cancer cells. Arch Pharm Res. 2004; 27:429–35.

24. Tian JM, Li H, Ye JM, Guo WF, Li LY, Wang LP. Effect of ginsenoside Rg2 on chemical myocardial ischemia in rats. Zhongguo Zhong Yao Za Zhi. 2003; 28:1191–2.
Go to : 
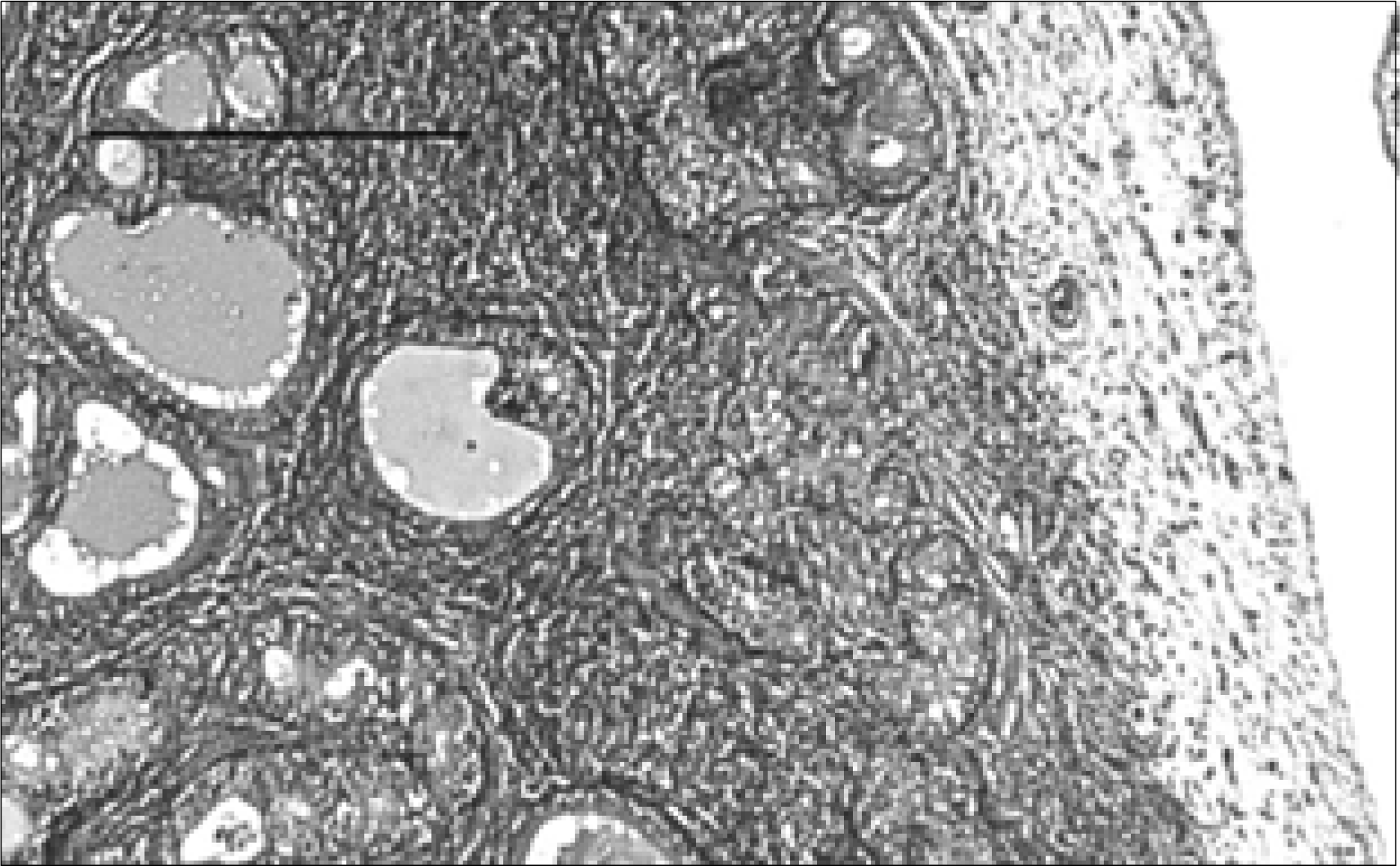 | Fig. 1.Prostate section of a rat with chronic bacterial prostatitis obtained 2 weeks after PBS treatment. The acinar structures are severely shrunken and obliterated. Marked chronic inflammatory cell infiltration and interstitial fibrosis are seen (H&E, Bar=200μm). PBS: phosphate buffered saline. |
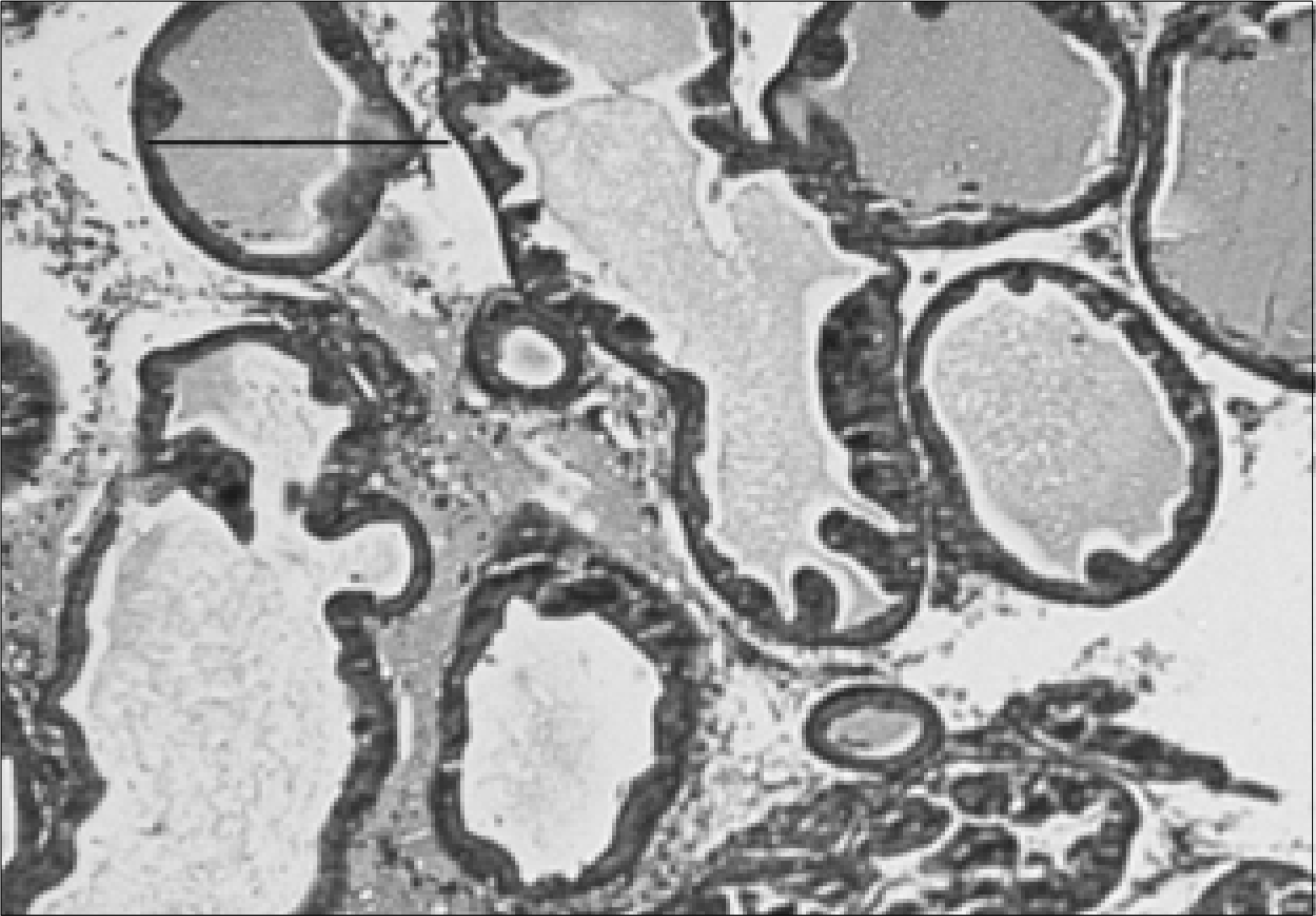 | Fig. 2.Prostate section of a rat with chronic bacterial prostatitis obtained 2 weeks after ciprofloxacin treatment. The acinar structures are partially atrophied, and mild lymphocytic infiltration, with partial interstitial fibrosis, is seen (H&E, Bar=200μm). |
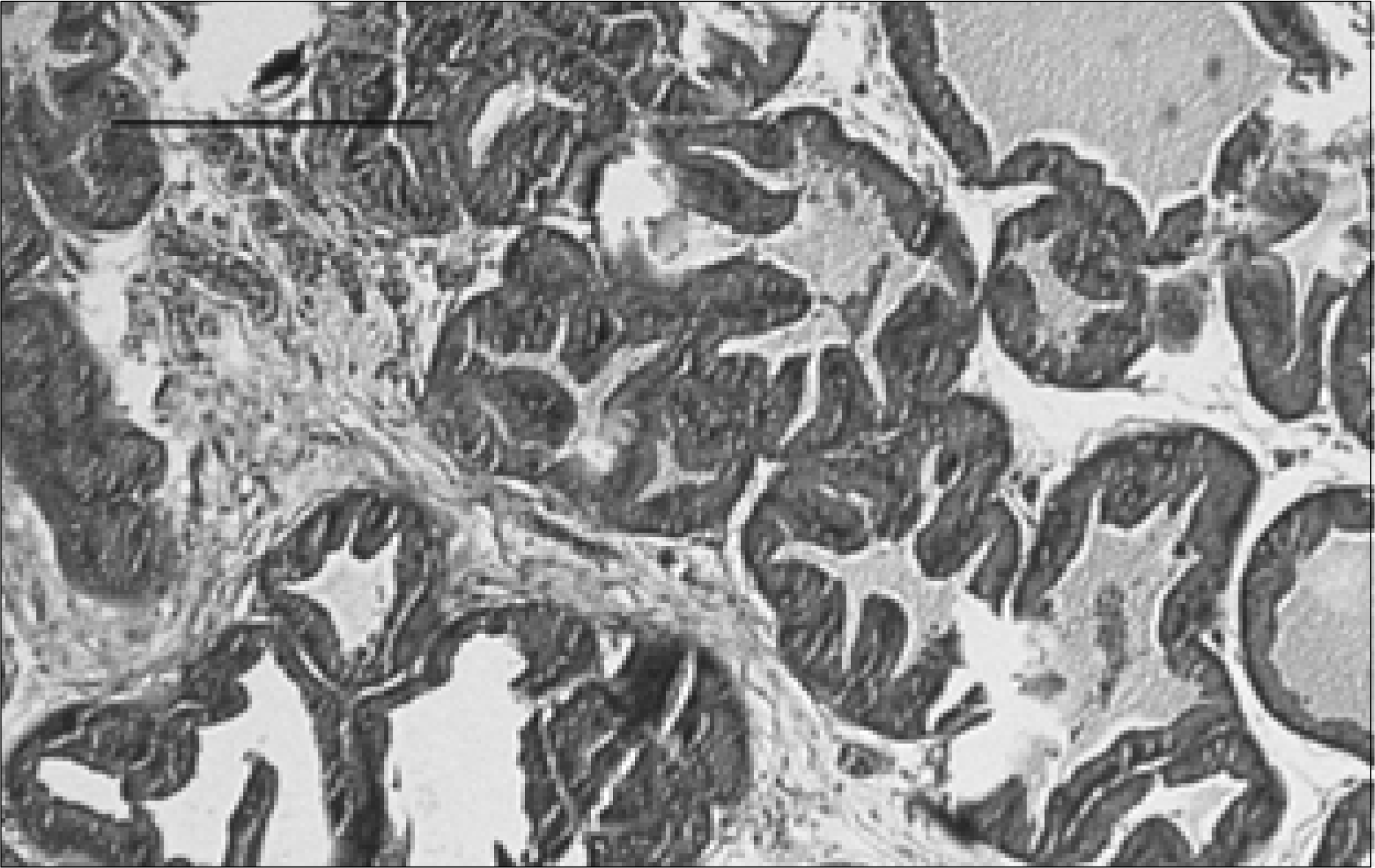 | Fig. 3.Prostate section of a rat with chronic bacterial prostatitis obtained 2 weeks after ginsenoside treatment. The acinar structures are moderately atrophied, shrunken and obliterated. Lymphocyte infiltration, with interstitial fibrosis, is clearly seen (H&E, Bar= 200μm). |
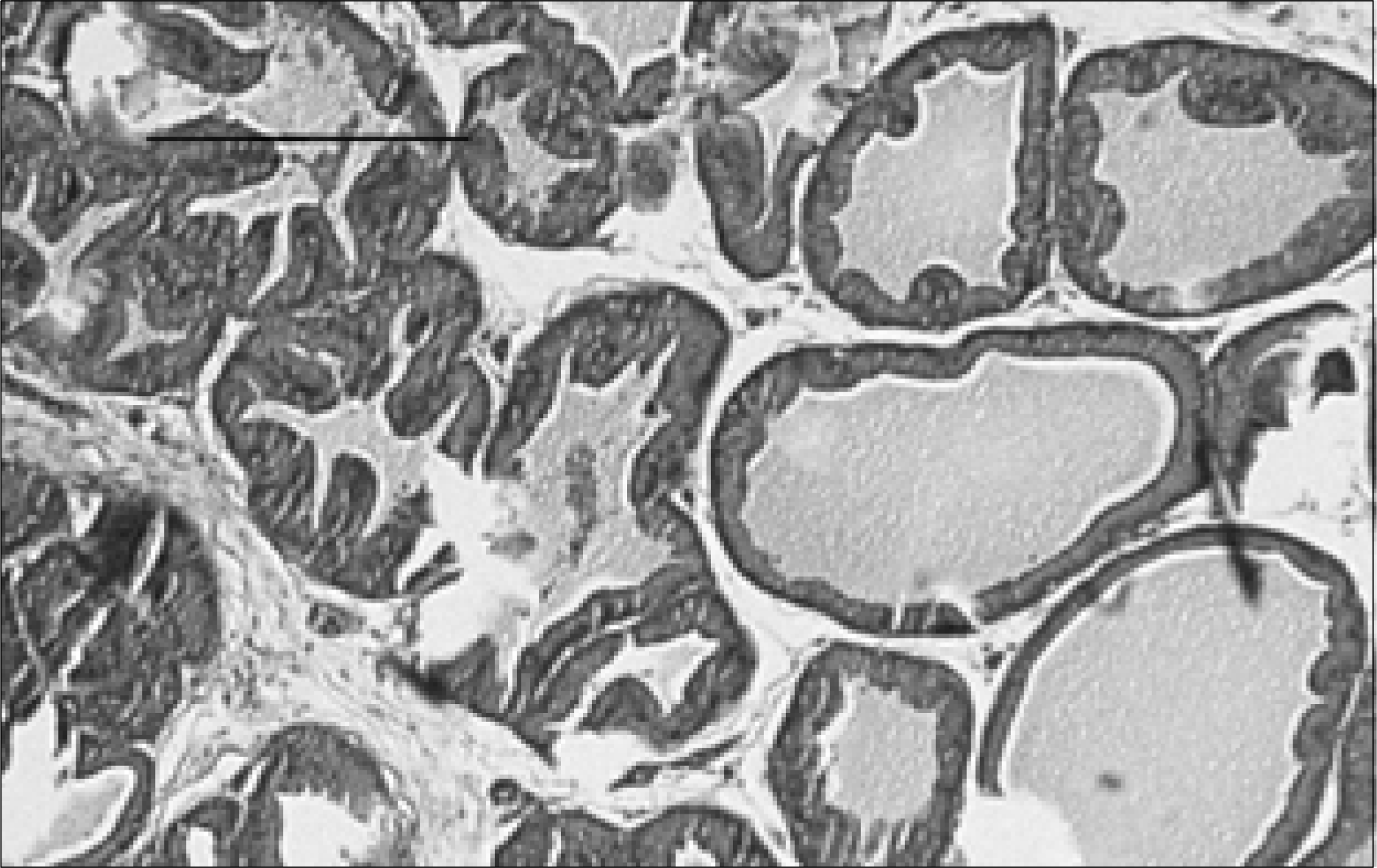 | Fig. 4.Prostate section of a rat with chronic bacterial prostatitis obtained 2 weeks after UrovaxomⓇ treatment. The acinar structures are moderately atrophied and obliterated. Chronic lymphocytic infiltration, with moderate interstitial fibrosis, is seen (H&E, Bar= 200μm). |
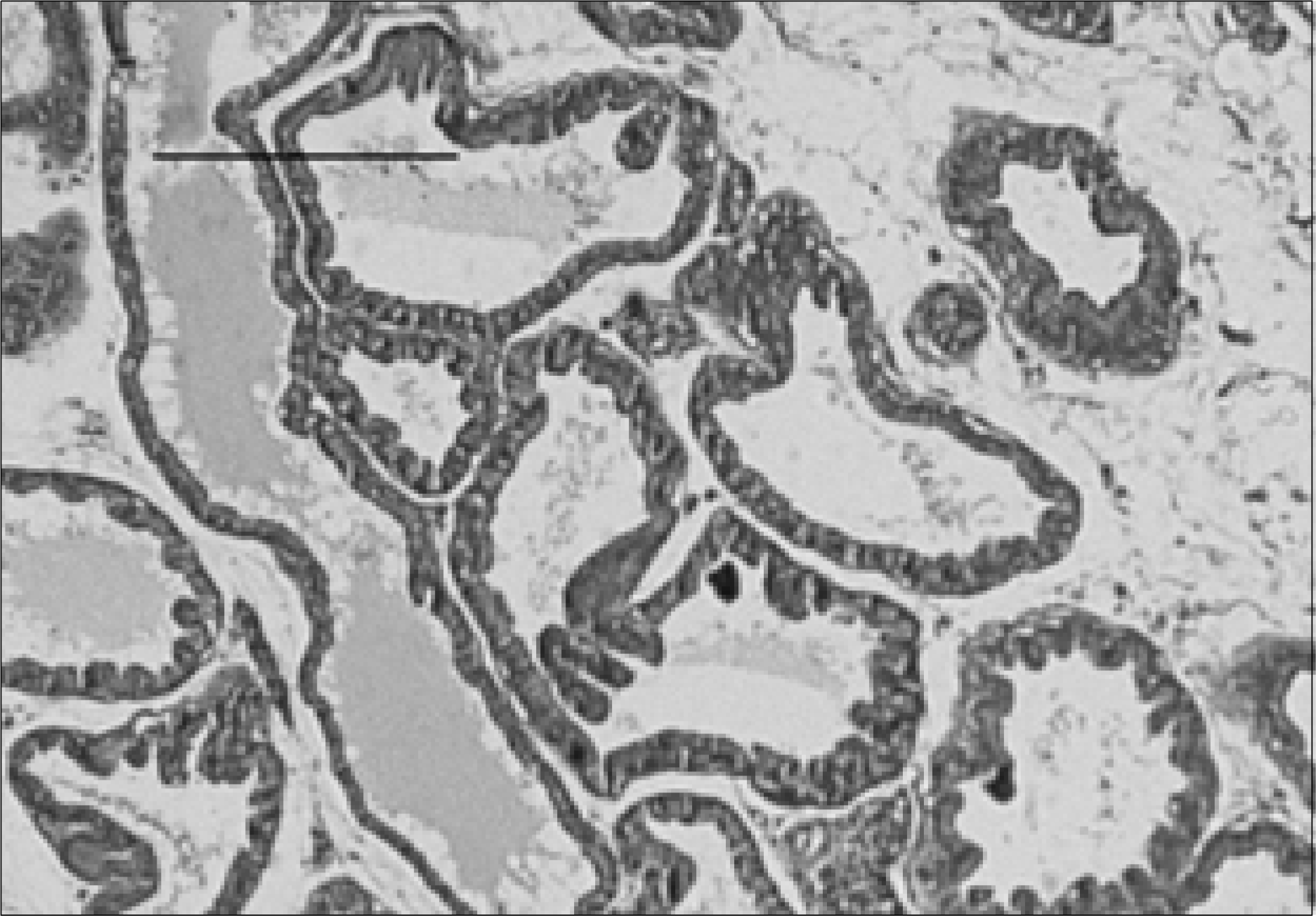 | Fig. 5.Prostate section of a rat with chronic bacterial prostatitis obtained 2 weeks after ginsenoside with ciprofloxacin treatment. The acinar structures are slightly atrophied and obliterated. A few chronic lymphocytic infiltrations, with minimal interstitial fibrosis, are seen (H&E, Bar=200μm). |
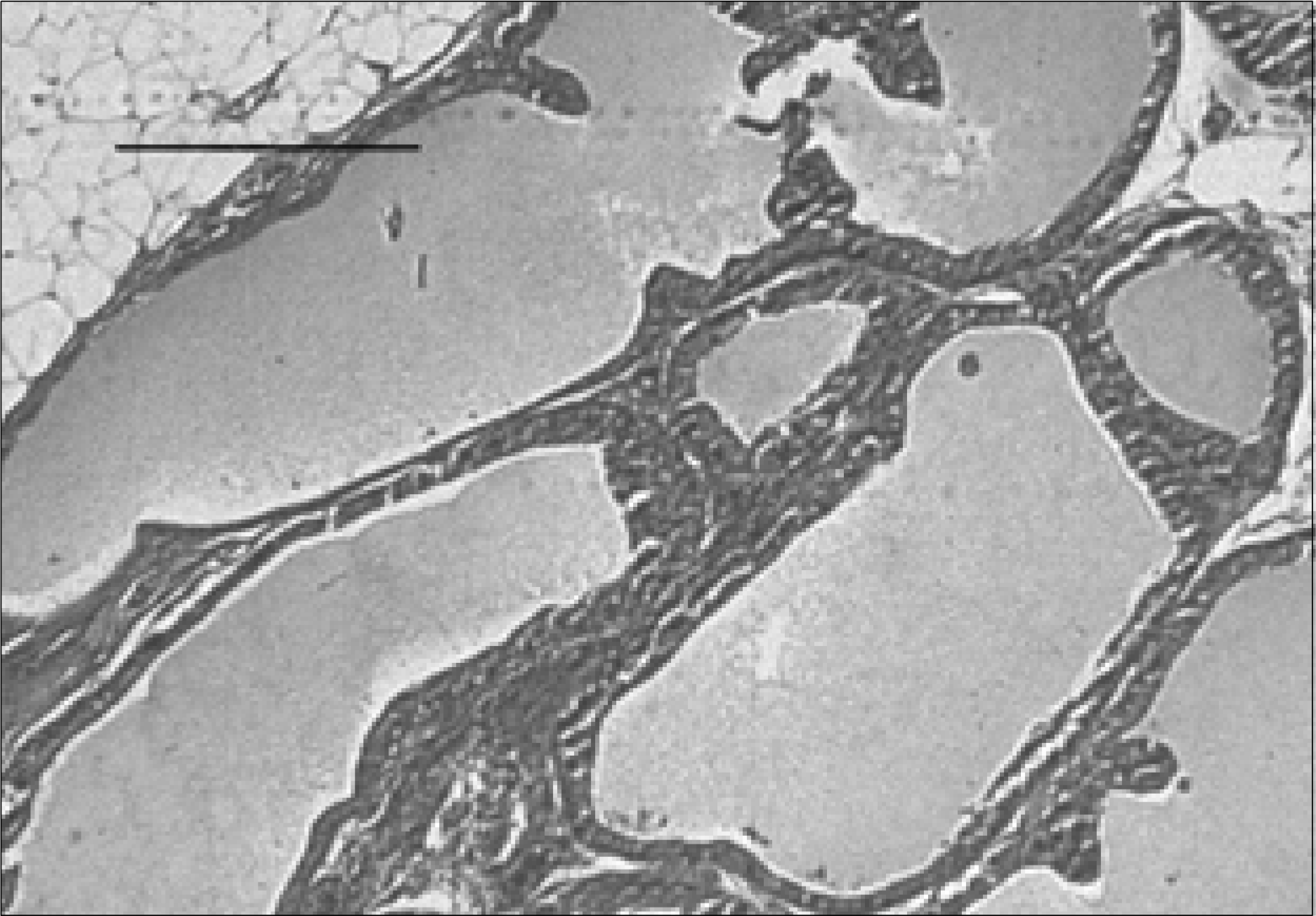 | Fig. 6.Prostate section of a rat with chronic bacterial prostatitis obtained 2 weeks after UrovaxomⓇ with ciprofloxacin treatment. The acinar structures are almost normal in appearance. Minimal interstitial fibrosis, with focal lymphocytic infiltrations, is seen (H&E, Bar=200μm). |
Table 1.
Microbiological data of the prostate tissue and urine culture samples
Table 2.
Inflammatory cell infiltration of prostate tissue
Table 3.
Acinar cell structural change of prostate tissue
Table 4.
Interstitial fibrosis with structural change in prostate tissue




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download