Abstract
Purpose
We evaluated the significance of the P504S expression in prostate cancer and also the usefulness of the P504S/34βE12 combined immunostaining method for diagnosing prostate cancer, and we did this by performing histological analysis of needle biopsy specimens.
Materials and Methods
Prostate tissue specimens were obtained from 83 patients with clinically suspected prostate cancer. A total of 54 prostate needle biopsy specimens were immunostained with an enzyme commonly overexpressed in prostate cancer (P504S) and also with an antibody against a basal cell marker (34βE12). A total of 83 cases were immunostained with 34βE12, including 29 cases that were stained with only with HMWCK (34βE12).
Results
P504S immunostaining was positive in 96.3% (26 of 27 cases) of the prostate cancer specimens. 34βE12 immunostaining was positive in 97.2% (35 of 36 cases) of the benign prostate tissues. Of the 30 P504S positive immunostaining cases, 26 cases were prostate cancers, 3 cases were ASAP and 1 case was ASAP+PIN. Of the 36 34βE12 positive immunostained cases, 35 cases were benign and 1 case was ASAP. In the P504S (+)/34βE12 (-) cases, there are no benign prostate lesions. There are no benign prostate lesions in the P504S (-)/34βE12 (+) cases, and all the cases were benign. There were no statistical correlations between the grade of P504S staining and the clinical parameters such as serum PSA, the clinical stage and the Gleason scores.
Figures and Tables
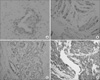 | Fig. 1P504S staining of the human prostate (A) P504S negative staining (×400) specimens at the intracytoplasm, (B) P504S weak staining (×200) of the intracytoplasm, (C) P504S moderate staining (×200) of the intracytoplasm and (D) P504S strong staining (×100) of the intracytoplasm. |
 | Fig. 234βE12 staining of the human prostate (A) 34βE12 negative staining (×100) specimens at the basement membrane and (B) 34βE12 positive staining (×200) of the basement mambrane. |
References
1. Green R, Epstein JI. Use of intervening unstained slides for immunohistochemical stains for high molecular weight cytokeratin on prostate needle biopsies. Am J Surg Pathol. 1999. 23:567–570.
2. Zhou M, Jiang Z, Epstein JI. Expression and diagnostic utility of alpha-methylacyl-CoA-racemase (P504S) in foamy gland and pseudohyperplastic prostate cancer. Am J Surg Pathol. 2003. 27:772–778.
3. Jiang Z, Woda BA, Rock KL, Xu Y, Savas L, Khan A, et al. P504S: a new molecular marker for the detection of prostate carcinoma. Am J Surg Pathol. 2001. 25:1397–1404.
4. Park WH, Lee SL, Gong GY, Ahn HJ. Role of basal cell and secretory cell in benign prostate hyperplasia and prostatic cancer. Korean J Urol. 1997. 38:386–392.
5. Rubin M, Zhou M, Dhanasekaran SM, Varambally S, Barrette TR, Sanda MG, et al. Alpha-methylacyl coenzyme A racemase as a tissue biomarker for prostate cancer. JAMA. 2002. 287:1662–1670.
6. Leite KR, Mitteldorf CA, Camara-Lopes LH. Repeat prostate biopsies following diagnoses of prostate intraepithelial neoplasia and atypical small gland proliferation. Int Braz J Urol. 2005. 31:131–136.
7. Luo J, Zha S, Gage WR, Dunn TA, Hicks JL, Bennett CJ, et al. Alpha-methylacyl-CoA racemase: a new molecular marker for prostate cancer. Cancer Res. 2002. 62:2220–2226.
8. Gown AM, Vogel AM. Monoclonal antibodies to human intermediate filament proteins. II. Distribution of filament proteins in normal human tissues. Am J Pathol. 1984. 114:309–321.
9. McCulloch DR, Opeskin K, Thompson EW, Williams ED. BM18: a novel androgen-dependent human prostate cancer xenograft model derived from a bone metastasis. Prostate. 2005. 65:35–43.
10. Battifora H, Kopinski M. The influence of protease digestion and duration of fixation on the immunostaining of keratins. A comparison of formalin and ethanol fixation. J Histochem Cytochem. 1986. 34:1095–1100.
11. Gown AM, Vogel AM. Monoclonal antibodies to intermediate filament proteins of human cells: unique and cross-reacting antibodies. J Cell Biol. 1982. 95:414–424.
12. Gown AM, Vogel AM. Monoclonal antibodies to human intermediate filament proteins. III. Analysis of tumors. Am J Clin Pathol. 1985. 84:413–424.
13. Grignon DJ, Ro JY, Ordonez NG, Ayala AG, Cleary KR. Basal cell hyperplasia, adenoid basal cell tumor, and adenoid cystic carcinoma of the prostate gland: an immunohistochemical study. Hum Pathol. 1985. 19:1425–1433.
14. Hedrick L, Epstein JI. Use of keratin 903 as an adjunct in the diagnosis of prostate carcinoma. Am J Surg Pathol. 1989. 13:389–396.
15. Fleshman RL, MacLennan GT. Immunohistochemical markers in the diagnosis of prostate cancer. J Urol. 2005. 173:1759.
16. Molinie V, Fromont G, Sibony M, Vieillefond A, Vassiliu V, Cochand-Priollet B, et al. Diagnostic utility of a p63/alpha-methyl-CoA-racemase (P504S) cocktail in atypical foci in the prostate. Mod Pathol. 2004. 17:1180–1190.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download