Abstract
Purpose
To compare the efficacy and safety of a 3-day regimen of extended-release ciprofloxacin (ciprofloxacin ER), 500mg qd, with trimethoprim-sulfamethoxazole (TMP/SMX), 800mg/160mg bid, for the empirical treatment of acute uncomplicated cystitis in Korean women.
Materials and Methods
A randomized, single-blind treatment trial of 75 women with acute uncomplicated cystitis was conducted. The women were prescribed ciprofloxacin ER, 500mg qd, or TMP/SMX, 800mg/160 mg, bid for 3 days. The patients were assessed in terms of the clinical and microbiological outcome and safety 7 days after treatment.
Results
Sixty-five women were eligible for the analyses (32 ciprofloxacin ER and 33 TMP/SMX). The most prevalent causative organism was Escherichia coli (76.9%), followed by Proteus (6.2%) and coagulase-negative Staphylococcus (6.2%). The rates of in vitro susceptibility to ciprofloxacin and TMP/SMX were 86.2 (56/65) and 73.4% (48/65), respectively. The clinical cure rates with ciprofloxacin ER and TMP/SMX were 87.5 and 78.8%, respectively. Microbiological cures at 7 days were observed in 25 of the 32 (78.1%) with ciprofloxacin ER and 18 of the 33 (54.5%) with TMP/SMX. The mean interval to improvement in the clinical symptoms after ciprofloxacin and TMP/SMX medications were 1.93±0.55 and 2.92±0.48 days, respectively. Adverse events with ciprofloxacin and TMP/SMX occurred in 28.1 and 15.5%, respectively, but both treatments were well tolerated.
Conclusions
Although some organisms were resistant (13.8%) to ciprofloxacin, ciprofloxacin ER was superior to TMP/SMX in terms of the clinical and microbiological cure rates and the mean interval to improvement in the clinical symptoms. The high prevalence of resistance and low microbiological cure rates for TMP/SMX suggest that this drug does not provide an adequate initial therapy, while once-daily ciprofloxacin ER was safe and effective in the empirical treatment of symptomatic uncomplicated cystitis.
Urinary tract infections (UTIs) are the most common bacterial infections in women. Many individuals suffer from symptomatic UTIs, worldwide each year.1 In clinical practice, the empirical management of uncomplicated cystitis is to use antimicrobials effective against most Escherichia coli (E. coli) strains, which account for around 80% of cases and are the predominant uropathogens, until the pathogens are confirmed in urine culture. The standard treatment of symptomatic uncomplicated cystitis is trimethoprim/sulfamethoxazole (TMP/SMX) or ciprofloxacin for 3 days.2 Recently, however, an increase has been observed in urinary tract pathogens resistant to the first-line antimicrobial agents-TMP/SMX.3 Ciprofloxacin has an efficacy similar to TMP/SMX. The mean susceptibilities to ciprofloxacin and TMP/SMX were 84.8% and 61.3% in a multicenter study of the antimicrobial susceptibility of uropathogens in Korea.4 Once-daily extended-release ciprofloxacin (ciprofloxacin ER) has been approved for the treatment of UTIs. Therefore, we compared the efficacy and safety of a 3-day regimen of ciprofloxacin ER 500mg qd with TMP/SMX 800mg/160mg bid in the empirical treatment of acute cystitis in Korean women, in a randomized, single-blind trial.
The study was conducted from August to December 2005 and was a randomized, single-blind trial. Women were eligible for enrollment if they were healthy, between 18 and 65 years old, and had dysuria, urgency, or frequency, with or without suprapubic pain or gross hematuria, and the absence of flank pain or fever >38℃. Women were ineligible for enrollment if they had a history of major allergy to fluoroquinolone or penicillin, a known anatomic or functional abnormality of the urinary tract, or had received systemic antimicrobials within the previous 14 days.
At the initial visit, the participants underwent a directed history and physical examination, a midstream urinalysis, urine culture, and liver and renal function tests. The women received either ciprofloxacin ER (500mg qd) or TMP/SMX (800mg/160 mg bid) for 3 days. They were asked to return to the clinic after 1 week and the tests performed at the initial visit were repeated at the second visit.
The primary objective was to determine drug efficacy based on clinical outcome, which was defined as the absence of symptoms. The secondary goals were to determine drug efficacy based on microbiological cure at the first posttreatment visit (1 week), the mean interval to improved clinical symptoms after medication, and adverse effects. The urine culture results were classified as positive (greater than at least 100CFU/ml) or negative (less than 100CFU/ml). We assessed the clinical and microbiological outcomes and safety 7 days posttreatment.
Clinical cure was defined as the absence of symptoms and microbiological cure was defined as the absence of a uropathogen or at least a 1-log drop in the colony count compared to the urine culture at enrollment.
Statistical analyses: the comparison in the two treatment groups were used chi-square test and Fisher's exact test. p<0.05 was considered statistically significant.
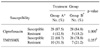
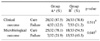
Seventy-five women were enrolled and randomized to receive treatment. Sixty-five women qualified for the analyses (32 randomized to receive ciprofloxacin ER and 33 randomized to receive TMP/SMX) (Table 1). Ten women were excluded from the analyses because of a uropathogen count less than 100 CFU/ml at enrollment. The most prevalent causative organism was E. coli (76.9%), followed by Proteus (6.2%), coagulase-negative Staphylococcus (6.2%), and Pseudomonas (4.6%) (Table 2). The mean in vitro susceptibility to ciprofloxacin ER and TMP/SMX was 86.2% (56/65) and 73.4% (48/65), respectively (Table 3). Clinical cures were observed in 28 (87.5%) of the 32 women treated with ciprofloxacin ER compared to 26 (78.8%) of the 33 treated with TMP/SMX. Microbiological cure at 7 days was observed in 25 (78.1%) of the 32 women treated with ciprofloxacin ER compared to 18 (54.5%) of the 33 women treated TMP/SMX (Table 2, 4). The rates of eradication of E. coli in Group A and B were 20/23 (86.9%), 16/27 (59.3) retrospectively (p=0.056, Fisher's exact test). The mean interval to improved clinical symptoms after medication was 1.93±0.55 days in the patients treated with ciprofloxacin ER compared to 2.92±0.48 days for those receiving TMP/SMX (p<0.01).
Adverse events were seen 28.1% (9/32) of those receiving ciprofloxacin ER versus 15.5% (5/33) for TMP/SMX (p=0.203). Gastrointestinal effects were the most common adverse events, accounting for 8 (88.8%) and 4 (80%) of the events, respectively. The remaining patient receiving ciprofloxacin ER complained of headache, while one patient receiving TMP/SMX had miscellaneous complaints. The liver and renal function test results (AST, ALT, BUN, Cr) remained unchanged.
This study found that a once-a-day regimen of ciprofloxacin ER for the empirical treatment of acute cystitis was more effective than twice-a-day TMP/SMX in 65 Korean women.
As women today are participating more in society and the work force, the issue of convenience regarding drug regimens is attracting greater attention. Convenience plays an important role that should not be discounted because UTIs commonly affect women during the busiest and most productive period of their lives. The convenience of administration is one of the most important factors improving patient compliance, optimizing the time for cure, preventing relapse, and facilitating medical treatment. Since UTI is the most common infectious disease in women, such a commonplace malady should be cured using the most comfortable and convenient regimen possible. E. coli remains the predominant uropathogen (75-90%) causing uncomplicated UTIs, followed by Staphylococcus (10-15%), Klebsiella, Enterobacter, and Proteus species.5 In Korea, similar findings were seen in a multicenter study on the antimicrobial susceptibility of uropathogens causing acute uncomplicated cystitis in 2003.4 In our study, the proportion of patients with E. coli was 83%, which matches other results.
In the treatment of acute uncomplicated UTI, empirical therapy should be started before the results of urine culture and antimicrobial susceptibility are available. The decision to manage uncomplicated cystitis is guided by the physician's perception of the symptom severity experienced by the patient, and by the highly predictable microorganisms and relatively predictable susceptibility to antimicrobials in local areas. The Infectious Diseases Society of America (IDSA) guidelines recommend that when resistance to TMP/SMX is less than 10-20%, the standard first-line treatment of uncomplicated UTIs should involve empirical therapy with a 3-day regimen of TMP/SMX or TMP alone for patients with sulfa or fluoroquinolone allergies.2 The IDSA guidelines also state that a 3-day fluoroquinolone regimen should be used for UTIs when the community resistance among uropathogens exceeds 10-20%.2 The efficacy and safety of a once-a-day ciprofloxacin extended-release (ciprofloxacin ER) formulation was compared to the standard twice-a-day TMP/SMX for 3 days based on the IDSA guidelines for the first-line therapy of acute cystitis.
The respective rates of resistance to TMP/SMX and ciprofloxacin were 38.7% and 15.2% in a Korean study conducted in 2003.4 The resistance to TMP/SMX is clearly higher than that to ciprofloxacin, so the traditional standard therapy for uncomplicated cystitis should be reconsidered. Although TMP/SMX is among the most active treatments of all those currently approved, with the highest resistance rate, TMP/SMX might no longer be the drug of choice for UTI in Korea.
At the 1 week follow-up, 87.5% of the women treated with ciprofloxacin ER had a clinical cure compared to 78.8% of those given TMP/SMX. In addition, ciprofloxacin ER was superior to TMP/SMX at eradicating uropathogens. Fortunately, E. coli remains susceptible to fluoroquinolones and nitrofurantoin, which have an efficacy analogous to that of TMP/SMX. However, since a 7-day regimen of nitrofurantoin is recommended, it is limited as a first-line treatment for uncomplicated UTI. Fluoroquinolones, including ciprofloxacin, norfloxacin, ofloxacine, and levofloxacin, have high cure rates and low resistance.6 The efficacy of these antimicrobials is reported to be similar to that of TMP/SMX when given as a 3-day regimen.7 We found a significant difference between the two drug treatments in terms of the mean interval to improved clinical symptoms. Patients experiencing a clinical improvement with ciprofloxacin ER were better at 1.93±0.55 days compared to patients given TMP/SMX (2.92±0.48 days, p<0.01). Therefore, based on the improved symptoms, ciprofloxacin ER is an appropriate empirical therapy for acute cystitis.
In women with uncomplicated cystitis, treatment-related adverse events were reported in 27.3% of the patients who received ciprofloxacin ER 500mg qd in 23.5% of those treated with the immediate-release 250mg bid formulation.8 Most of the adverse events were mild or moderate in severity.5 In our study, no serious adverse events occurred in either group. Of the adverse events to ciprofloxacin ER, 8/9 had mild nausea and 1/9 had a headache. Laboratory changes, including serum AST, ALT, BUN, and creatinine, have been reported, although none were seen in our study. No treatment discontinuation occurred due to adverse reactions thought to be drug-related.
Considering its efficacy and safety, ciprofloxacin ER is an advance in terms of convenience and an effective first-line drug for uncomplicated UTI in Korean women. Ciprofloxacin ER thus provides another antimicrobial option for women with acute uncomplicated cystitis.
The high prevalence of resistance and low microbiological cure rates with TMP/SMX suggest this drug does not provide adequate initial therapy, and once-daily ciprofloxacin ER was safe, effective, and sufficiently convenient compared to twice-daily TMP/SMX in the empirical treatment of symptomatic uncomplicated cystitis.
Figures and Tables
References
1. Stamm WE, Hooton TM. Management of urinary tract infections in adults. N Engl J Med. 1993. 329:1328–1334.
2. Warren JW, Abrutyn E, Hebel JR, Johnson JR, Schaeffer AJ, Stamm WE. Guidlines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infections Diseases Society of America (IDSA). Clin Infect Dis. 1999. 29:745–758.
3. Gupta K, Scholes D, Stamm WE. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA. 2000. 281:736–738.
4. Lee SJ, Cho YH, Kim BW, Lee JG, Jung SI, Lee SD, et al. A multicenter study of antimicrobial susceptibility of uropathogens causing acute uncomplicated cystitis in woman. Korean J Urol. 2003. 44:697–701.
5. Talan DA. Ciprofloxacin extended-release and urinary tract infection. 2003. New Jersey: Science Press;5–8.
6. Talan DA, Naber KG, Palou J, Elkharrat D. Extended-release ciprofloxacin (Cipro XR) for treatment of urinary tract infections. Int J Antimicrob Agents. 2004. 23:S54–S66.
7. Stamm WE. Scientific and clinical challenges in the management of urinary tract infections. Am J Med. 2002. 113:Suppl 1A. 1S–4S.
8. Henry DC Jr, Bettis RB, Riffer E, Haverstock DC, Kowalsky SF, Manning K, et al. Comparison of once-daily extended-release ciprofloxacin and conventional twice-daily ciprofloxacin for the treatment of uncomplicated urinary tract infection in women. Clin Ther. 2002. 24:2088–2104.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download