Abstract
Purpose:
To investigate the locations of the cerebral cortex activated by visually stimulated sexual arousal, and to discriminate the gender differences between the cortical activation patterns in response to sexual stimuli.
Materials and Methods:
Thirty-two male and the twenty-one female v㢌unteers from right-handed medical students were enr㢌led in this study. 丁he electroencephalography (EEGs) included the segments recorded during resting, watching a music-video, intermission and watching a pornographic video. 丁he low-res㢌ution brain electromagnetic tomography (LORE丁A) images of cross-spectral analysis were obtained from the segments using the LORE丁A-KEY software.
Results:
The beta 1, 2 and 3 activities of males showed the point of maximal current densities in both the uncus and paramppocampal gyrus of the left limbic lobe, the anterior cingulate of the right limbic lobe, the superior temporal gyrus of both temporal lobes, the precuneus of the right parietal lobe, the medial frontal gyrus and superior frontal gyrus of the right frontal lobe, the superior parietal lobule of the right parietal lobe, and the middle occipital gyrus of both occipital lobes. 丁he delta, theta, alpha and beta 1 activities of females showed the point of maximal current densities in the postcentral gyrus and inferior parietal lobule of the left parietal lobe, the middle frontal gyrus of the left frontal lobe, the middle occipital gyrus of the left occipital lobe, the left cuneus, the superior temporal gyrus of both temporal lobes and the left parahippocampal gyrus.
Conclusions:
There was a difference in the visually stimulated sexual arousal-associated with the cerebral neuroanatomical areas between men and women, as estimated using the LORE丁A software. 丁hese areas; therefore, were thought to play important r㢌es in the sexual arousal of males and females in response to audiovisual sexual stimulation.
Go to : 
REFERENCES
1.Shepard GM. Neurobiology. 3rd ed.New York: Oxford University Press;1994. ;3-12.
3.Merboldt KD., Fransson P., Bruhn H., Frahm J. Functional MRI of the human amygdala? Neuroimage. 2001. 14:253–7.

4.Koukounas E., McCabe M. Sexual and emotional variables influ-encing sexual response to erotica. Behav Res Ther. 1997. 35:221–30.

5.Meston CM. Sympathetic nervous system activity and female sexual arousal. Am J Cardiol. 2000. 86:30–4.

6.McKenna K. The brain is the master organ in sexual function: central nervous system control of male and female sexual function. Int J Impot Res. 1999. 11(Suppl l):S48–55.
7.Bancroft J. Human sexuality and its problems. 2nd ed Edinburgh: Churchill Livingstone;. 1989. 748.
8.Herbert J. Sexuality, stress and the chemical architecture of brain. Annu Rev Sex Res. 1996. 7:1–43.
9.Pascual-Marqui RD., Michel CM., Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol. 1994. 18:49–65.

10.Kim MR., Kim KR., Ha CK., Choi SH., Lee IK. Comparative study between visual analysis and low resolution electromagnetic tomography (LORETA) method in the localizaion of epileptiform discharges. J Korean Neurol Assoc. 2002. 20:164–8.
11.Talairach J., Toumoux P. Co-planar stereotaxic atlas of die human brain. 1st ed.New York: Thieme Stuttgart;1988. p. 1.
12.Towle VL., Bolanos J., Suarez D., Tan K., Grzeszczuk R., Levin DN, et al. The spatial location of EEG electrodes: locating the best-fitting sphere relative to cortical anatomy. Electroencephalogr Clin Neurophysiol. 1993. 86:1–6.

13.Karama S., Lecours AR., Leroux JM., Bourgouin P., Beaudoin G., Joubert S, et al. Areas of brain activation in males and females during viewing of erotic film excerpts. Hum Brain Mapp. 2002. 16:1–13.

14.Stoleru S., Gregoire MC., Gerard D., Decety J., Lafarge E., Cinotti L, et al. Neuroanatomical correlates of visually evoked sexual arousal in human males. Arch Sex Behav. 1999. 28:1–21.
15.Bocher N., Chisin R., Parag Y., Freedman N., Meir Weil Y., Lester H, et al. Cerebral activation associated with sexual arousal in response to a pornographic clip: a 150-H20 PET study in heterosexual men. Neuroimage. 2001. 14:105–17.
16.Grafton ST., Arbib MA., Fadiga L., Rizzolatti G. Localization of grasp representations in humans by positron emission tomography. 2. Observation compared with imagination. Exp Brain Res. 1996. 112:103–11.

17.Phelps ME., Mazziotta JC. Positron emission tomography: human brain function and biochemistry. Science. 1985. 228:799–809.

18.Ogawa S., Lee TM., Kay AR., Tank DW. Brain magnetic resonance imaging widi contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990. 87:9868–72.
19.Amow BA., Desmond JE., Banner LL., Glover GH., Solomon A., Polan ML, et al. Brain activation and sexual arousal in healthy, heterosexual males. Brain. 2002. 125:1014–23.
20.Beauregard M., Levesque J., Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001. 21:RC165.

21.Park K., Seo JJ., Kang HK., Ryu SB., Kim HI., Jeong GW. A new potential of blood oxygenation level dependent (BOLD) functional MRI for evaluating cerebral centers of penile erection. Int J Impot Res. 2001. 13:73–81.

22.Park KS., Kang HK., Seo JJ., Kim HJ., Ryu SB., Jeong GW. Blood-oxygenation-level-dependent functional magnetic resonance imaging for evaluating cerebral regions of female sexual arousal response. Urology. 2001. 57:1189–94.

23.Mourns H., Stoleru S., Bittoun J., Glutron D., Pelegrini-Issac M., Paradis AL, et al. Brain processing of visual sexual stimuli in healthy men: a functional magnetic resonance imaging study. Neuroimage. 2003. 20:855–69.

24.Mulert C., Jager L., Schmitt R., Bussfeld P., Pogarell O., Moller HJ, et al. Integration of fMRI and simultaneous EEG: towards a comprehensive understanding of localization and time-corse of brain activity in target detection. Neuroimage. 2004. 22:83–94.
25.Joseph R. The limbic system: emotion, laterality, and unconscious mind. Psychoanal Rev. 1992. 79:405–56.
26.Dagi TF., Poletti CE. Reformulation of the Papez circuit: absence of hippocampal influence on cingulate cortex unit activity in the primate. Brain Res. 1983. 259:229–36.

27.Mumen SK., Stockton M. Gender and self-reported sexual arousal in response to sexual stimuli: a meta-analytic review. Sex Roles. 1997. 37:135–53.
28.Mesulam MM. Pattern in behavioral neuroanatomy: association areas, the limbic system, and behavioral specialization. Mesulam MM, editor. editor.Principles of behavioral neurology. 1st ed.Philadelphia: FA Davis;1985. p. 1–20.
Go to : 
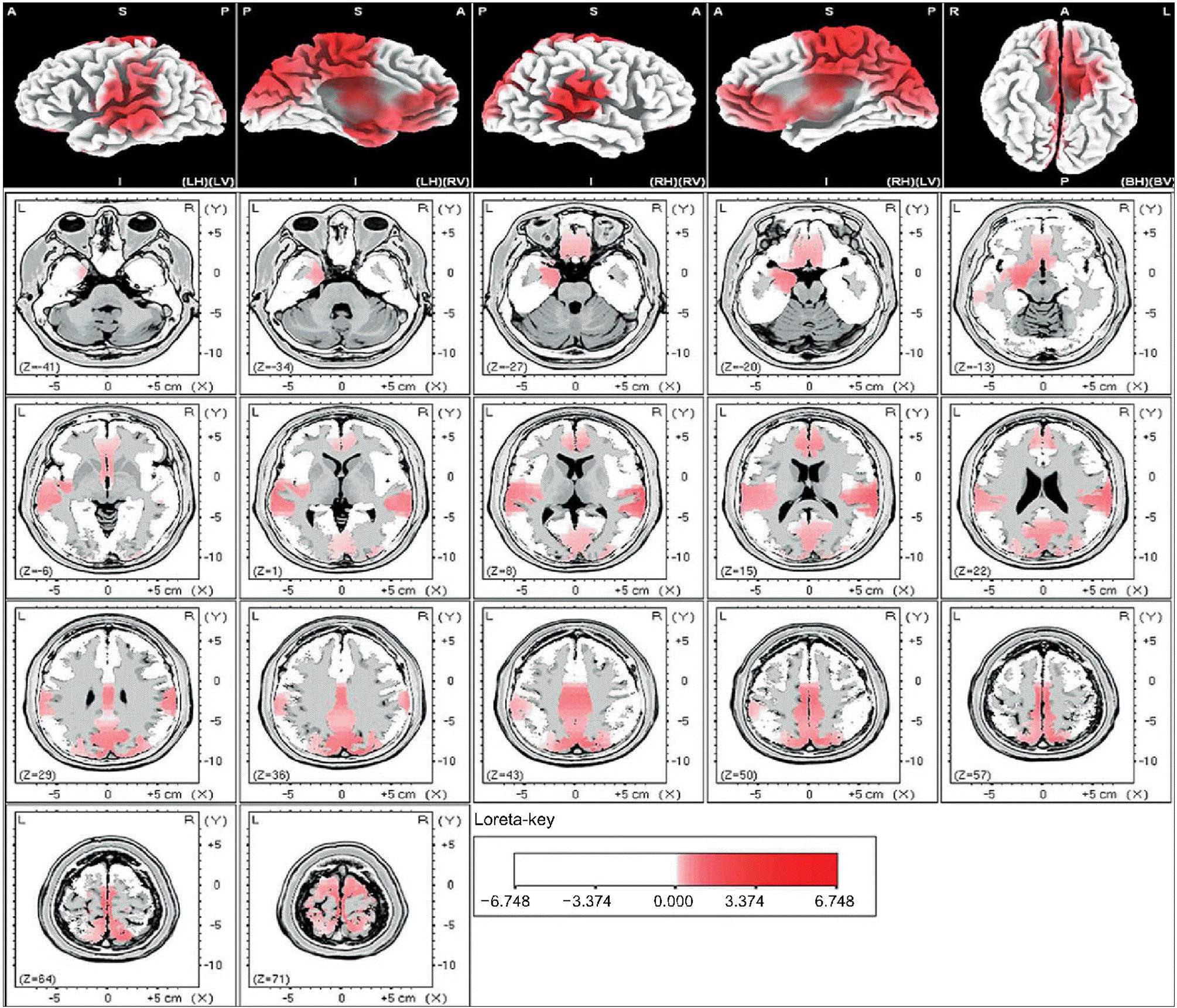
 | Fig. 1.Male-beta 1. The anatomical areas of current density increment of the beta 1 (13-18Hz) band of male induced by visual-sexual arousal. The current density is increased in the parahippocampal gyrus, and uncus of the left limbic lobe, superior temporal gyrus of both temporal lobes and the middle occipital gyrus of the left occipital lobe. |
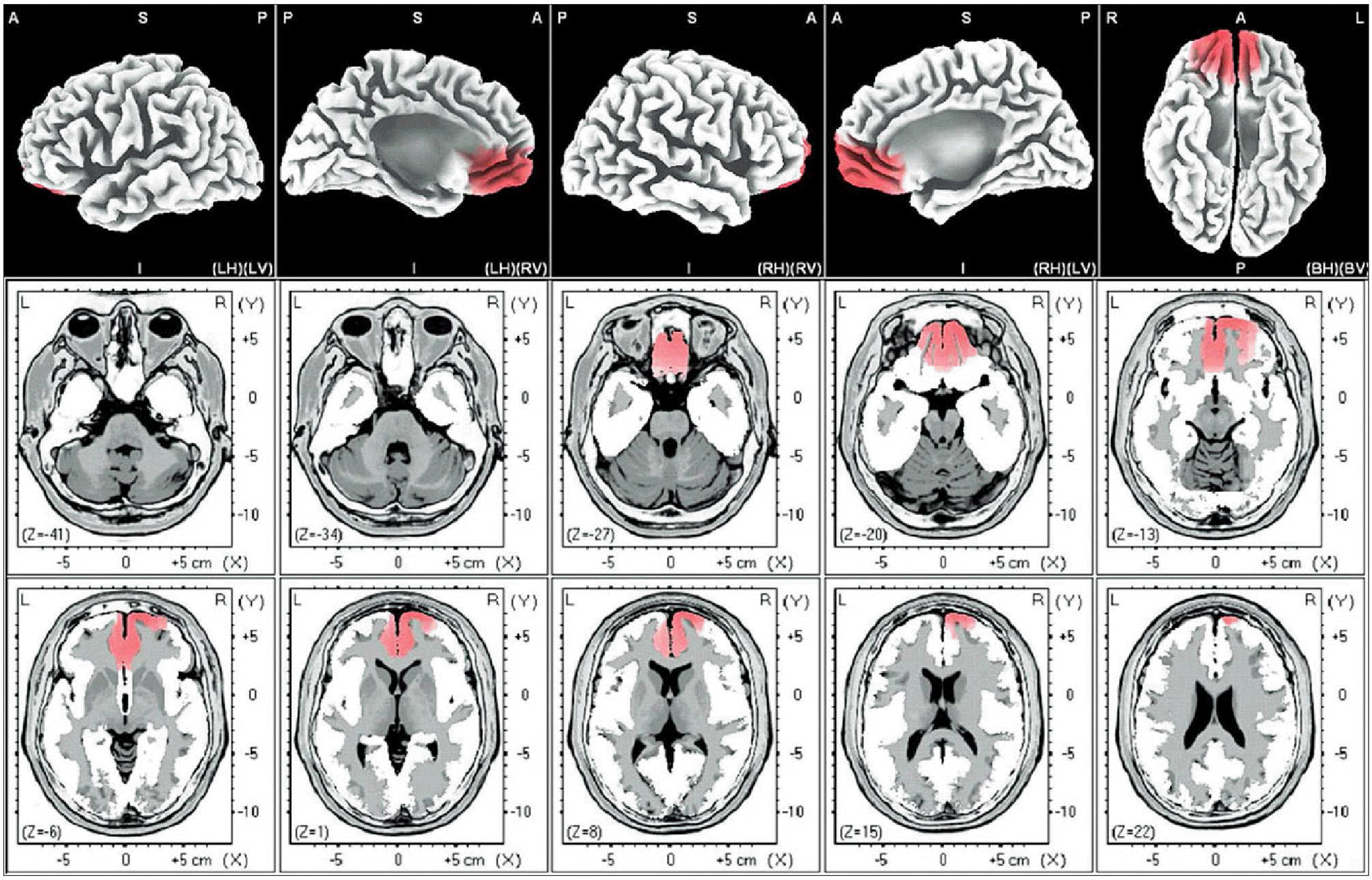 | Fig. 2.Male-beta 2. The anatomical areas of current density increment of the beta 2 (19-21Hz) band of male induced by visual-sexual arousal. The current density is increased in the superior frontal gyrus and medial frontal gyrus of the right frontal lobe, and the anterior cingulate gyrus of the left limbic lobe. |
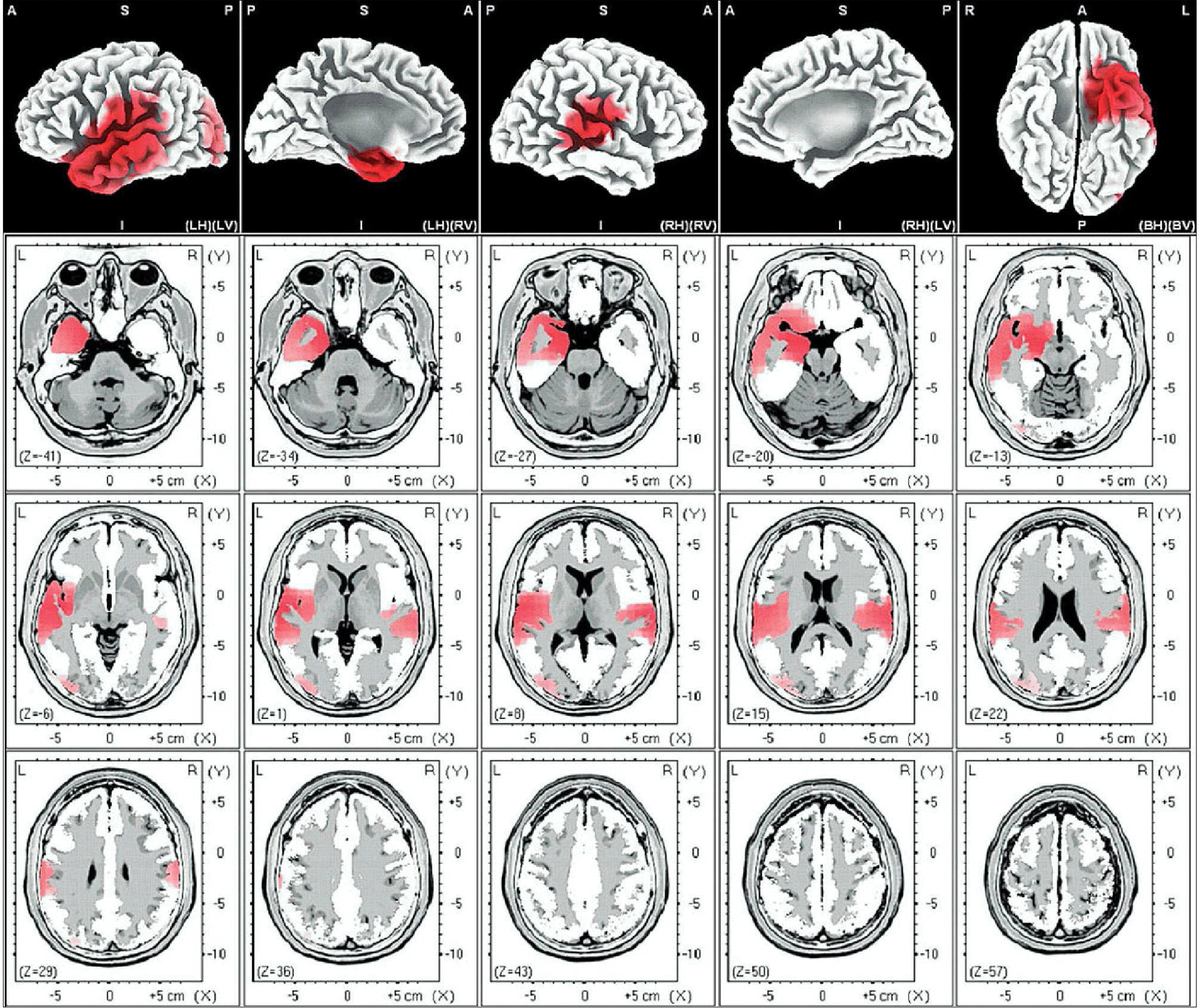
 | Fig. 3.Male-beta 3. The anatomical areas of current density increment of the beta 3 (22-30Hz) band of male induced by visual-sexual arousal. The current density is increased in the middle occipital gyrus of both occipital lobes, the superior temporal gyrus of both temporal lobes, the precuneus of the right parietal lobe, the superior parietal lobule of the right parietal lobe, the medial frontal gyrus and superior frontal gyrus of the right frontal lobe, the anterior cingulate gyrus of the right limbic lobe, and the parahippocampal gyrus of the left limbic lobe. |
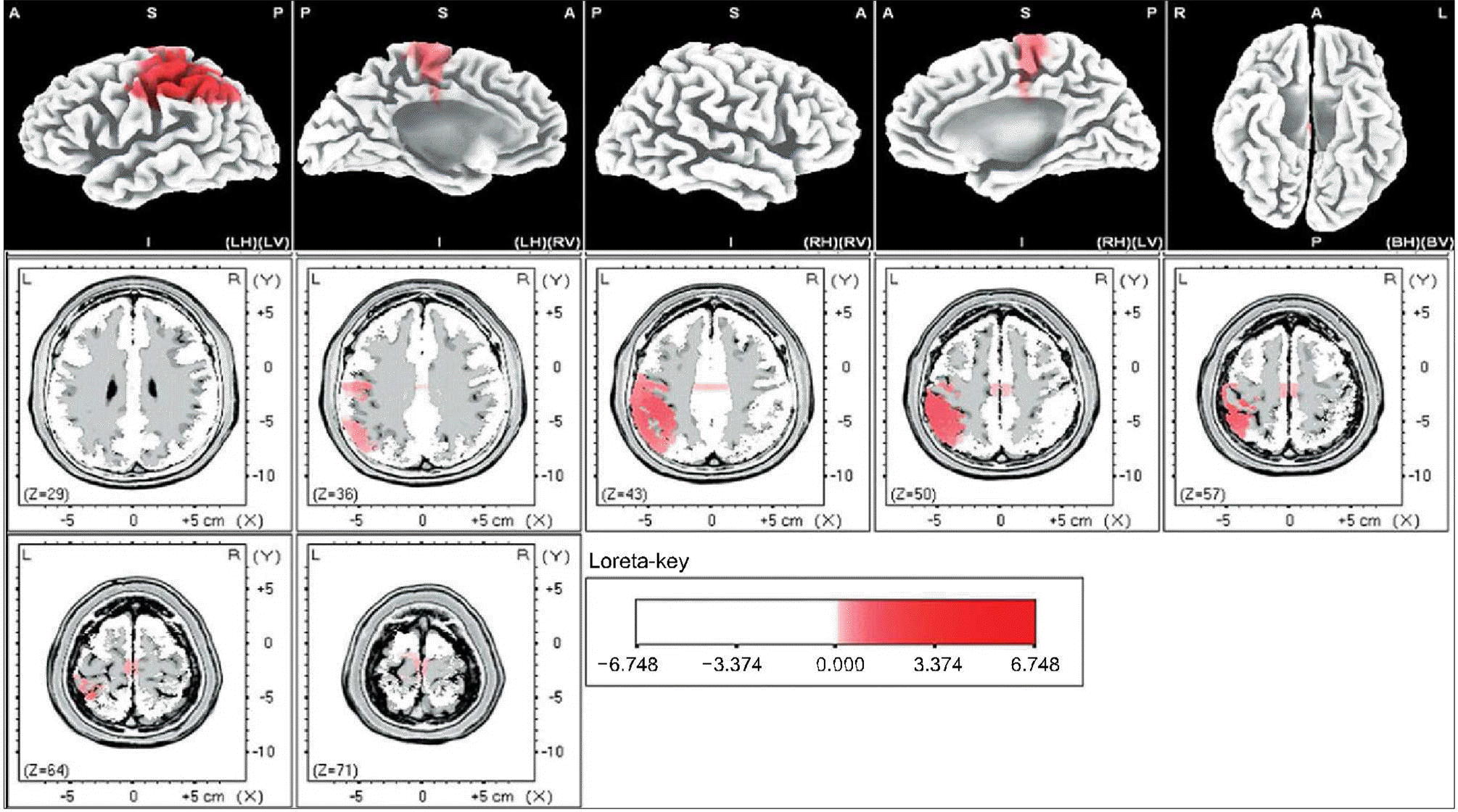 | Fig. 4.Female-delta. The anatomical areas of current density increment of the delta (1-3Hz) band of female induced by visual-sexual arousal. The current density is increased in the postcentral gyrus and inferior parietal lobule of the left parietal lobe, and the middle frontal gyrus of the left frontal lobe. |
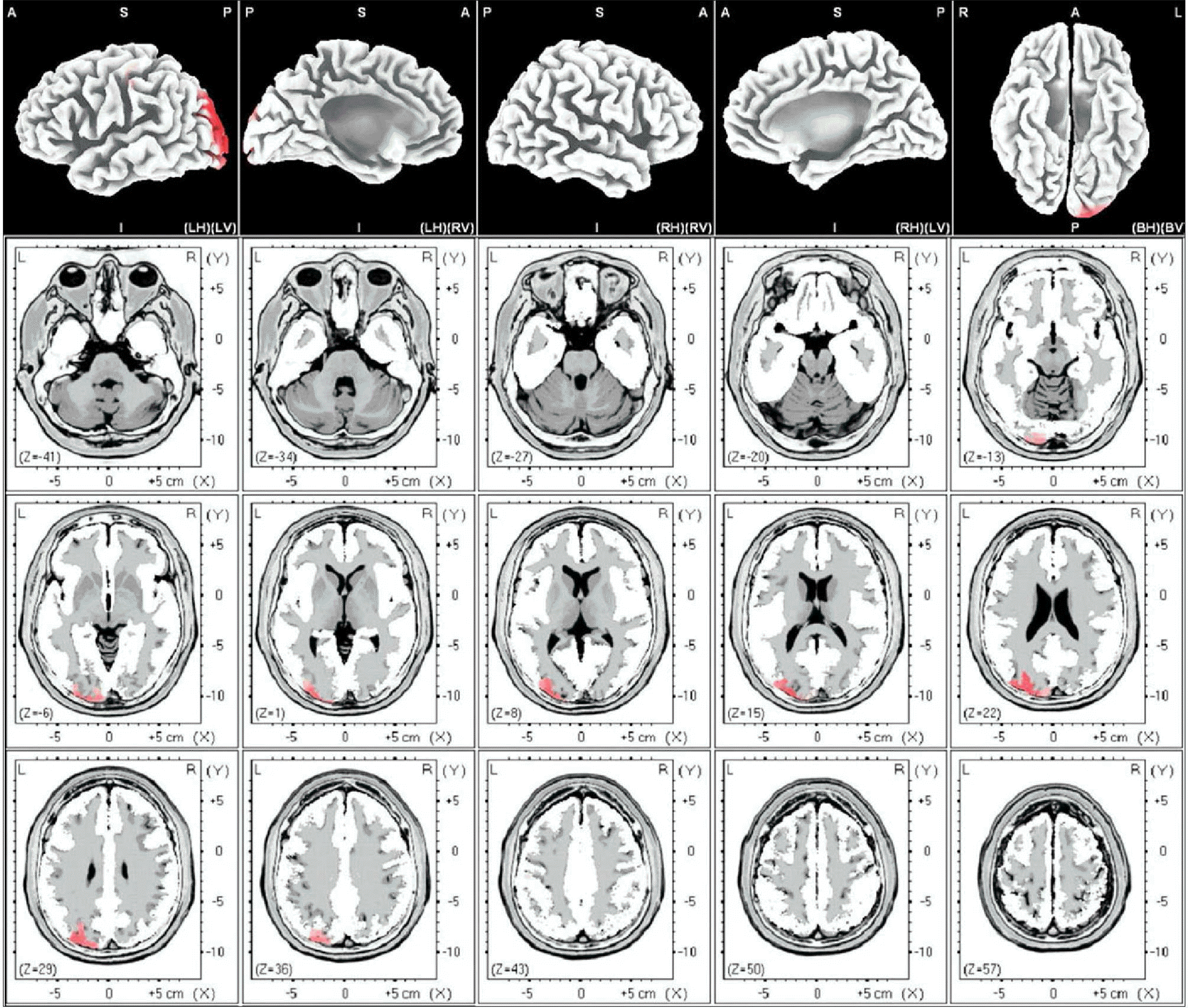 | Fig. 5.Female theta. The anatomical areas of current density increment of the theta (4-7Hz) band of female induced by visual-sexual arousal. The current density is increased in the middle occipital gyrus of the left occipital lobe, the left cuneus and the postcentral gyrus of the left parietal lobe. |
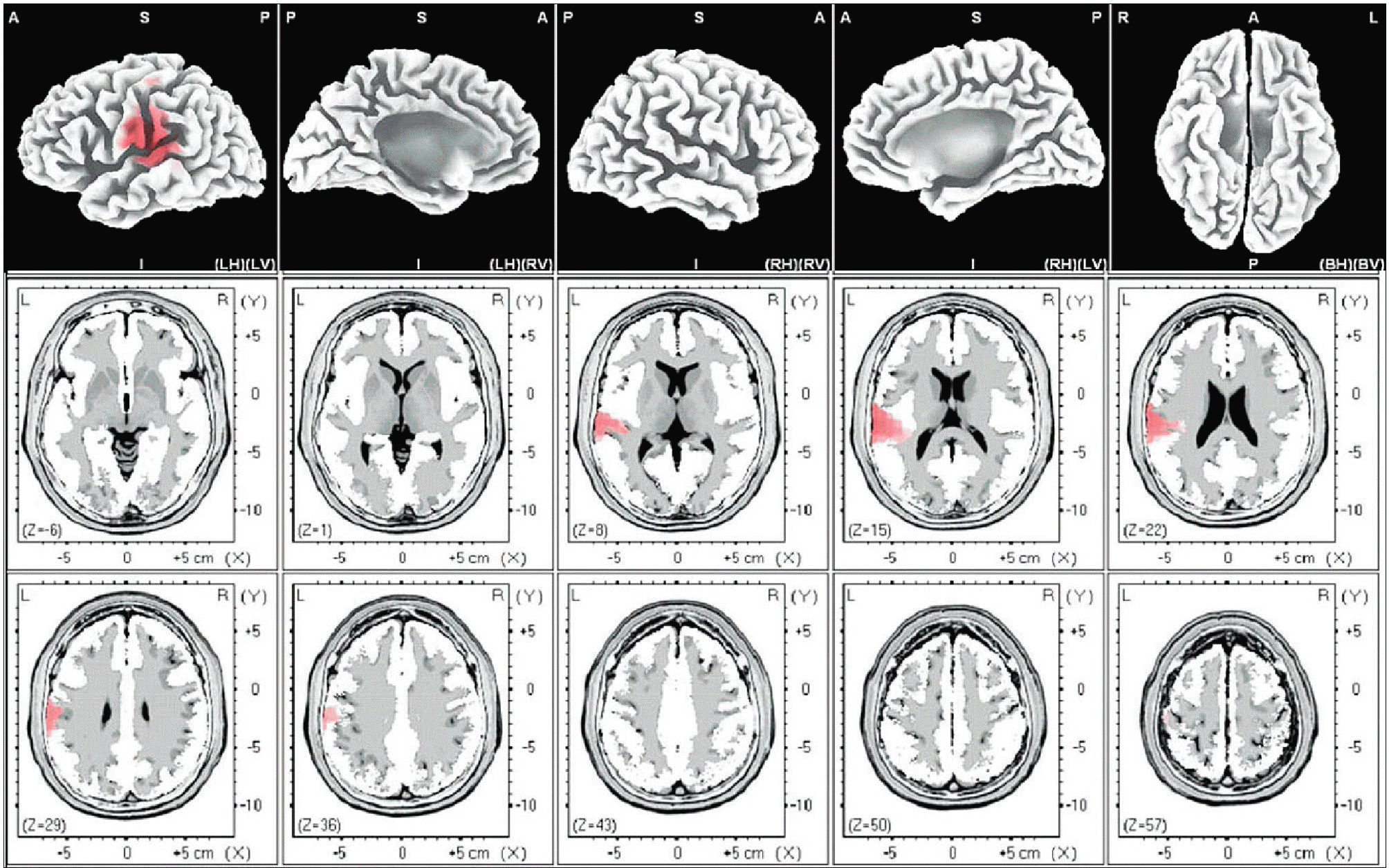 | Fig. 6.Female alpha. The anatomical areas of current density increment of the alpha (8-12Hz) band of female induced by visual-sexual arousal. The current density is increased in the superior temporal gyrus of the left temporal lobe, and the postcentral gyrus of the left parietal lobe. |
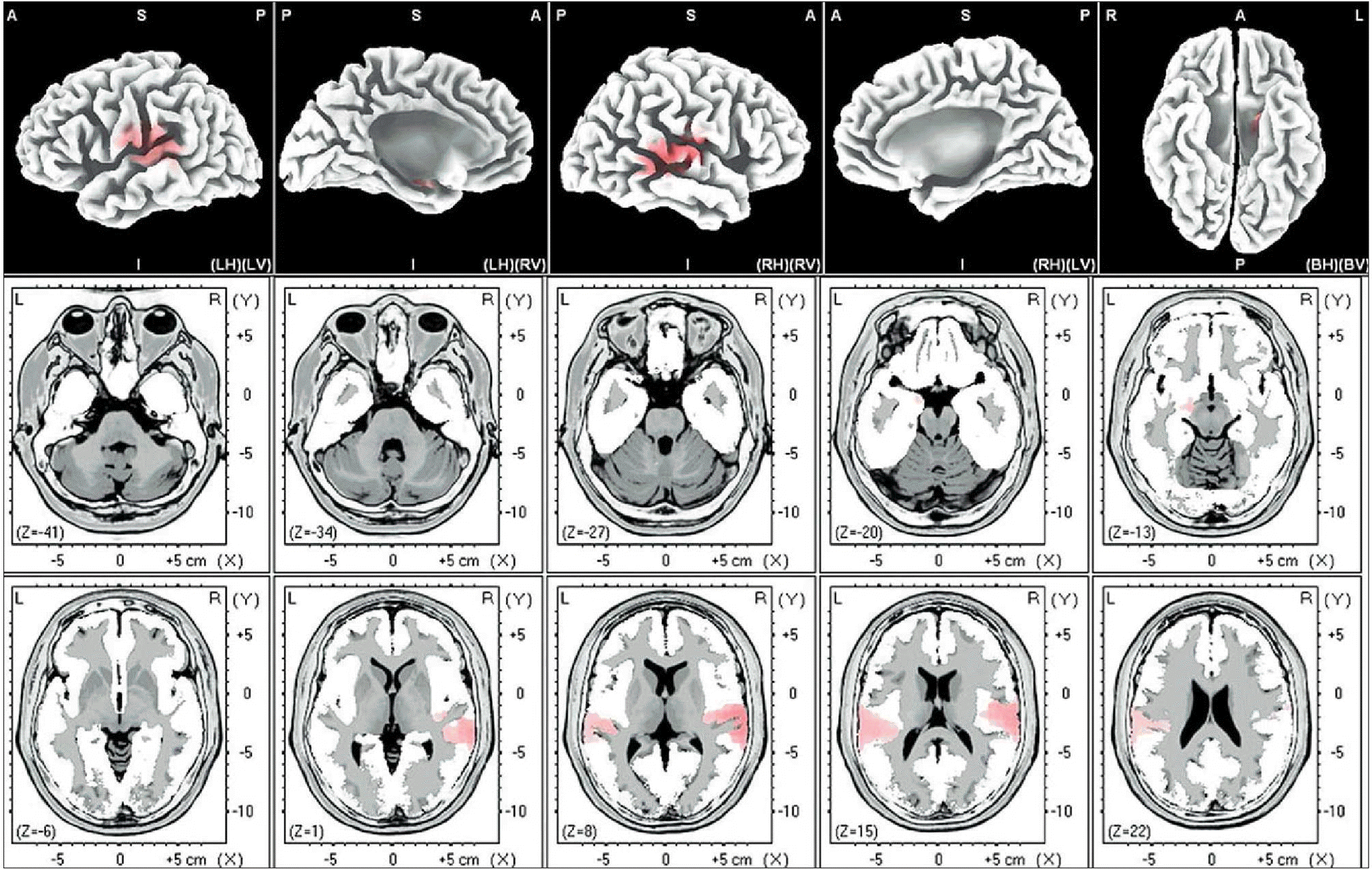 | Fig. 7.Female-beta 1. The anatomical areas of current density increment of the alpha (8-12Hz) band of female induced by visual-sexual arousal. The current density is increased in the superior temporal gyrus of both temporal lobes, the postcentral gyrus of the left parietal lobe, and the parahippocampal gyrus of the left limbic lobe. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download