Abstract
Purpose
Neuroendocrine (NE) cells in a prostate carcinoma may play important roles in tumor growth, proliferation and progression. The aim of this study was to evaluate the relationship between the NE cell differentiation status and pathological characteristics of prostate cancer.
Materials and Methods
Radical prostatectomy specimens from 215 patients were available for analysis. NE cell were detected by immunohistochemistry, using antibodies to chromogranin A (CgA). Tumor cell proliferation was assessed using the Ki-67 proliferation index (PI) employing the MIB-1 antibody. Staining of CgA was scored as: 0= no staining; 1= staining cell <10; 2= staining 10-20; and 3= staining cell >20. Tumors were classified depending on their staining score, positive staining and growth pattern.
Results
NE cell differentiation was present in 25.1% (54/215) of tumors. The amount of NE cells significantly increased; from tumors with solitary scattered NE cells to both small and large clusters (p<0.05). NE cell differentiation and the growth pattern were correlated with the Ki-67 PI (p<0.05). With respect to high-grade tumors, an increased PI was found in tumors with positive NE cells compared with those with negative NE (p<0.05). Pathologically advanced tumors, or those with higher histological grades, were associated with NE cell differentiation and Ki-67 PI (p<0.05).
Figures and Tables
Fig. 1
Immunohistochemical staining with chromogranin a shows neuroendocrine cell differentiation in neoplastic glands. (A) Immunostaining score: +1. (B) Immunostaining score: +2. (C) Immunostaining score: +3 (×100).

Fig. 2
Immunohistochemical staining with chromogranin A shows neuroendocrine cell differentiation in neoplastic glands. Tumors were categorized by the distribution patterns of neuroendocrine differentiation. (A) Neuroendocrine cells are scattered in the glands (no cluster). (B) Neuroendocrine cells are in small clusters. (C) Neuroendocrine cells are in large clusters (×100).
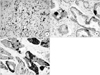
Fig. 3
Immunohistochemical staining with MIB-1 shows Ki-67 expression in neoplastic glands. (A) Low Ki-67 proliferation index: ≤6%. (B) High Ki-67 proliferation index: >6% (×400).

Table 1
Clinical characteristics of patients with prostate cancer after a radical prostatectomy (n=215)

Table 2
Serum PSA, histological grade, pathological stage and tumor volume in prostatic carcinoma (n=215) according to the neuroendocrine cells and Ki-67 proliferation index

Table 3
Mean proliferation index, as determined by Ki-67 expression, in the hot spot of neuroendocrine cell differentiation in prostatic carcinomas

References
1. di Sant'Agnese PA. Neuroendocrine differentiation in carcinoma of the prostate. Diagnostic, prognostic, and therapeutic implications. Cancer. 1992. 70(1):Suppl. 254–268.
2. Islam MA, Kato H, Hayama M, Kobayashi S, Igawa Y, Ota H, et al. Are neuroendocrine cells responsible for the development of benign prostatic hyperplasia? Eur Urol. 2002. 42:79–83.
3. di Sant'Agnese PA, Cockett AT. Neuroendocrine differentiation in prostatic malignancy. Cancer. 1996. 78:357–361.
4. Xing N, Qian J, Bostwick D, Bergstralh E, Young CY. Neuroendocrine cells in human prostate over-express the anti-apoptosis protein survivin. Prostate. 2001. 48:7–15.
5. Yu DS, Hsieh DS, Chang SY. Modulation of prostate carcinoma cell growth and apoptosis by chromogranin A. J Urol. 2003. 170:2031–2035.
6. Aprikian AG, Cordon-Cardo C, Fair WR, Reuter VE. Characterization of neuroendocrine differentiation in human benign prostate and prostatic adenocarcinoma. Cancer. 1993. 71:3952–3965.
7. Cohen RJ, Glezerson G, Taylor LF, Grundle HA, Naude JH. The neuroendocrine cell population of the human prostate gland. J Urol. 1993. 150:365–368.
8. Adlakha H, Bostwick DG. Paneth cell-like change in prostatic adenocarcinoma represents neuroendocrine differentiation: report of 30 cases. Hum Pathol. 1994. 25:135–139.
9. Allen FJ, Van Velden DJ, Heyns CF. Are neuroendocrine cells of practical value as an independent prognostic parameter in prostate cancer? Br J Urol. 1995. 75:751–754.
10. Pruneri G, Galli S, Rossi RS, Roncalli M, Coggi G, Ferrari A, et al. Chromogranin A and B and secretogranin II in prostatic adenocarcinomas: neuroendocrine expression in patients untreated and treated with androgen deprivation therapy. Prostate. 1998. 34:113–120.
11. Speights VO Jr, Cohen MK, Riggs MW, Coffield KS, Keegan G, Arber DA. Neuroendocrine stains and proliferative indices of prostatic adenocarcinomas in transurethral resection samples. Br J Urol. 1997. 80:281–286.
12. Abrahamsson PA. Neuroendocrine differentiation in prostatic carcinoma. Prostate. 1999. 39:135–148.
13. Bonkhoff H. Neuroendocrine cells in benign and malignant prostate tissue: morphogenesis, proliferation, and androgen receptor status. Prostate. 1998. 8:Suppl. 18–22.
14. Jiborn T, Bjartell A, Abrahamsson PA. Neuroendocrine differentiation in prostatic carcinoma during hormonal treatment. Urology. 1998. 51:585–589.
15. Grobholz R, Bohrer MH, Siegsmund M, Junemann KP, Bleyl U, Woenckhaus M. Correlation between neovascularisation and neuroendocrine differentiation in prostatic carcinoma. Pathol Res Pract. 2000. 196:277–284.
16. Bonkhoff H, Wernert N, Dhom G, Remberger K. Relation of endocrine-paracrine cells to cell proliferation in normal, hyperplastic, and neoplastic human prostate. Prostate. 1991. 19:91–98.
17. Isshiki S, Akakura K, Komiya A, Suzuki H, Kamiya N, Ito H. Chromogranin A concentration as a serum marker to predict prognosis after endocrine therapy for prostate cancer. J Urol. 2002. 167:512–515.
18. Cher ML, Chew K, Rosenau W, Carroll PR. Cellular proliferation in prostatic adenocarcinoma as assessed by bromodeoxyuridine uptake and Ki-67 and PCNA expression. Prostate. 1995. 26:87–93.
19. Grobholz R, Griebe M, Sauer CG, Michel MS, Trojan L, Bleyl U. Influence of neuroendocrine tumor cells on proliferation in prostatic carcinoma. Hum Pathol. 2005. 36:562–570.
20. Theodoropoulos VE, Tsigka A, Mihalopoulou A, Tsoukala V, Lazaris AC, Patsouris E, et al. Evaluation of neuroendocrine staining and androgen receptor expression in incidental prostatic adenocarcinoma: prognostic implications. Urology. 2005. 66:897–902.
21. Hirano D, Okada Y, Minei S, Takimoto Y, Nemoto N. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur Urol. 2004. 45:586–592.
22. Borre M, Stausbol-Gron B, Nerstrom B, Overgaard J. Immunohistochemical BCL-2 and Ki-67 expression predict survival in prostate cancer patients followed expectantly. Prostate Cancer Prostatic Dis. 1998. 1:268–275.
23. Pretl K. Frage der Endokrinie der menschleichen Vorsteherdruse. Virchows Arch Pathol Anat Physiol Klin Med. 1994. 312:392–404.
24. Lee JJ, Choi HY, Lee NK. Expression of endocrine cells in the prostate of rat and guinea pig after orchiectomy. Korean J Urol. 2006. 45:1148–1155.
25. Hong SJ, Kwon SM, Kim SI, Oh HY, Chung BC. Expression of neuroendocrine cells in benign prostatic hyperplasia and the effect of dihydrotestosterone. Korean J Urol. 2003. 44:267–271.
26. Sciarra A, Monti S, Gentile V, Salciccia S, Gomez AM, Pannunzi LP, et al. Chromogranin A expression in familial versus sporadic prostate cancer. Urology. 2005. 66:1010–1014.
27. Kim JM, Lee KW, Kim YH, Go ES, Kim ME, Lee NK. Expression pattern of neuroendocrine cells and survivin in the prostate of rabbits. Korean J Urol. 2006. 47:201–205.
28. Pollack A, DeSilvio M, Khor LY, Li R, Al-Saleem TI, Hammond ME, et al. Ki-67 staining is a strong predictor of distant metastasis and mortality for men with prostate cancer treated with radiotherapy plus androgen deprivation: Radiation Therapy Oncology Group Trial 92-02. J Clin Oncol. 2004. 22:2133–2140.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download