Abstract
Purpose
We wanted to evaluate the effects of a 2.45 GHz electromagnetic field (EMF) radiation on germ cell spermatogenesis.
Materials and Methods
Twenty male Sprague-Dawley rats (4 weeks of age) were exposed to a 2.45 GHz EMF for 1 hour or 2 hours a day. A sham-exposed group served as the control. The whole body average specific absorption rate (SAR) was 1.41 W/kg and the electric field intensity was 60.1mV/m. The rats were confined in cages specially designed for this study, and power was generated by a magnetron. After 8 weeks of exposure, the rats were sacrificed. The testicular germ cell status was assessed by histopathological examination and this was correlated with the hormonal level of the blood serum.
Results
Quantitative analysis of the Leydig cells showed a significantly higher count in the 2 hours exposed rats than in the sham controls (p<0.05), while the difference between the two exposed groups was insignificant. Moreover, a concomitant increase in the serum testosterone level was observed. A significantly decreased number of spermatocytes appeared at the seminiferous tubules in rats exposed for 1 and 2 hours, while this was not seen in the control.
Conclusions
These changes suggest that long-term exposure to EMF has adverse effects on the proliferation and differentiation of spermatogonia and this may be important in understanding the pathogenesis of EMF-induced male infertility. However, further studies are needed to investigate the effects of a longer exposure time and higher dose.
The rapid expansion of mobile phone and microwave oven utilization has given rise to widespread concern about the safety of electromagnetic field (EMF) exposure. According to experimental data, EMF might inhibit transmission of signals involved in the cell membrane (calcium ion), gene expression, genetic mutation, immune expression, neuro-endocrine system, and reproductive system.1 Among studies which focused on the reproductive effects of EMF (0.1 MHz-300 GHz) in animals, Kowalczuk et al.2 reported that 2.45 GHz microwave radiation at 44 W/kg for 30 min resulted in abnormal sperm morphology, reduced sperm count and primary spermatocyte. The experimental design that Kowalczuk et al.2 adopted corresponds to high density exposure for short term period. These type of EMF exposure rarely take place in everyday lives of ordinary people. However, we focused on to observe the long term effects of low density EMF. Thus, we chose specific absorption rate (SAR) level of 1.4 W/kg instead, which was similar to the amount of daily exposure for the general public and exposure limit in Korea and does not raise body temperature, and investigated whether it had an adverse effect on spermatogenesis when administered repeatedly at the pubertal stage. For the frequency of EMF, 2.45 GHz was selected because it is the frequency generated from human-made sources such as microwave ovens and mobile phones. The objective of this study is to investigate the effects of long term exposure to 2.45 GHz of EMF on reproductive function in male rats.
Three week-old male Sprague-Dawley rats weighing 150-200 g were obtained from Korea Institute of Toxicology (KRICT) and acclimatized at the mouse facility at the Yeungnam University College of Medicine for 1 week before the experiments. The rats were housed under 12 hour light/ 12 hour dark cycle, constant temperature (19-22℃) and 30-70% humidity and were fed distilled water and lab chow ad libitum. Experiments were performed in accordance with the Animal Experimentation Committee Regulation.
The EMF device and shield room was designed to irradiate the experimental groups of rat in equal amount and density of EMF by the Institute of biomedical engineering, Yeungnam University (Fig. 1). Power was generated by a magnetron (Samsung electronics, Korea) operating at 2.45 GHz. Forward power into and reflected power from the waveguide were measured by means of a 20dB coaxial bi-directional coupler and two power meters (Marconi Sanders 6460). For rats between a weight of 150 and 200 g, the time and whole-body averaged SAR has been determined to be 1.4 W/kg. This resembles the amount of daily exposure for the general public. Measurement of the EMF was performed at two locations in the chamber every week. The rats were housed in specially designed non-metallic polycarbonate cages fitted with non-metallic water bottles and placed on the tray of the EMF-exposure device.
Thirty rats were divided at random into 3 groups of 10 animals each. Two experimental groups were exposed to a 2.45 GHz EMF for 1 hour/day (Group E1) or 2 hours/day (Group E2) at 2 p.m. for 8 weeks. For sham-exposure, rats were placed for 2 hours in similar waveguides, but they were not irradiated. The body weight was recorded every week. Mean rectal temperature was measured before and after the microwave irradiation. After exposure to EMF, the rats were killed with a lethal dose of penthobarbital, intraperitoneally and both testes were excised and were weighed. Animal protocols used in the research reported here were approved by the Animal Care Committee of the School of Medicine, Yeungnam University College of Medicine.
To minimize the diurnal fluctuation of hormonal secretion, blood was drawn at between 9 a.m. and 11 a.m. by heart puncture and serum concentrations of luteinizing hormone (LH) and follicle stimulating hormone (FSH) were measured using a chemiluminescence assay, and the testosterone concentration was determined by radioimmunoassay.
The testes were removed for histopathological examination. The mean seminiferous tubular diameter was calculated for each H-E stained testis under light microscope. This was done by averaging the diameters of 40 round seminiferous tubules randomly selected in each tissue section. Tissues were examined histopathologically by using a testicular biopsy score count.3 The morphology and number of germ cells, Sertoli cells, and Leydig cells were also evaluated.
Semen samples were collected from the left caudal epididymis and were assessed for number and gross morphology without the investigator knowing which samples were from which group. The number of sperm was counted under a light microscope with a Neubauer hemocytometer (Paulmarine, Germany). The morphology of sperm were also examined under the light microscope using sperm smears.
There was no statistically significant difference in the body weight development between exposed and control groups. The mean rectal temperature after exposure was 33.2±0.2℃ in Group E1 and 33.3±0.1℃ in Group E2 compared to 32.1±0.5℃ in the sham exposed group. Overall, no significant differences were noted between the groups.
The mean testicular weight was 1.8±0.2 g in the control group, 1.8±0.5 g in Group E1 and 1.9±0.2 g in Group E2 showing no differences between the groups.
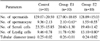
Table 1 shows the results of the micrometric parameter of the testis. The measured seminiferous tubular diameters of rats in the exposed groups were not statistically lower than the sham exposed group. There was no significant difference in the number of spermatids between the groups though a significant difference was seen in the number of spermatocytes between the control group and Groups E1 (p<0.05) and E2 (p<0.01). However, no significant difference was noted between Groups E1 and E2 themselves. There was no significant difference in the Sertoli cell count between the groups. The number of Leydig cells in Group E2 was significantly higher than in the control group (p<0.05). In the control group, the average testicular biopsy score was 9.83±0.08, whereas in the Group E1 and E2, they were slightly lower (9.24±0.05 and 9.12±0.23, respectively) and there were no statistically significant differences (Fig. 2).
As shown in Table 2, epididymal sperm count in group E2 was slightly less than controls, but the difference was not statistically significant. Also, there were no sperms showing abnormal morphology in any of the groups.
Serum testosterone levels differed significantly between the groups with values of 1.1±0.5 ng/ml and 2.6±1.4 ng/ml for the control and Group E2, respectively (p<0.01). In Group E1, serum testosterone increased to 1.8 (0.8 ng/ml. However, no statistically significant change was observed compared with the control group. The levels of serum LH and FSH did not show any significant difference between the groups (Fig. 3).
Conflicting observations have been reported on the adverse effects of EMF on spermatogenesis and reproductive function. Among studies reporting the potential toxic effects, Saunders and Kowalczuk4 and Kowalczuk et al.2 have shown that 1.7 GHz (50 mW/cm2), 2.45 GHz (44 W/kg and 30 W/kg) can affect seminiferous epithelium, sperm count, sperm morphology, and primary spermatocytes in mice. However, these studies focused mainly on the thermal effects of EMF.
Hyperthermia clearly increases the incidence of infertility. However, low intensity pulsed EMF radiation currently emitted by cell phones or microwave ovens exert non-thermal influences on living organisms long before heating occurs with the temperature rise no more than 0.1℃.5 Circumstances that would raise the body temperature would be rarely encountered by the general population.6 The biological effects from EMF alone were to be investigated in this research. Hyperthermic effects of EMF exposure are observed when the absorbed energy per kg body weight per second (called the specific absorption rate, SAR) exceeds about 4 W/kg for periods of about 1 hour. Also, EMF in the frequency range of 10 MHz to 300 GHz is reported to have non-thermal effects on organisms. Thus, we chose 2.45 GHz EMF (SAR of 1.41 W/kg) in the absence of a discernible rise in temperature during exposure.
Nagler and White7 reported that in male rats, spermatogenesis begins 15 days after birth and they are fertile 45 days after birth. In our study, the irradiation had been initiated at sexually immature 4-weeks, even before spermatozoa appeared in the seminiferous epithelium and was sustained to 8 weeks. There are many doubts about how long is long enough to observe the long term effects of EMF, though, Hecht and Balzer8 reported that the long term effects have been given from 200 hours up to 20 years in the study of EMF effects.
Many of the activities associated with reproduction including meiosis, fertilization, and implantation of the embryo are especially sensitive to toxic influences. The high rate of cell division and differentiation in the developing fetus and in the seminiferous epithelium make it particularly vulnerable. These results are reproduced where significant degeneration of seminiferous epithelium in mice was observed after 50 mW/cm2 (1.7 GHz) EMF radiation.4,9,10 One of our main findings is that the spermatocyte count was the lowest in the testis of Group E2. This number was statistically significantly lower than that of the Group E1 and the control. Our data confirms the results obtained by Saunders and Kowalczuk,4 who reported that spermatocytes were the most sensitive to 2.45 GHz microwave radiation.
In spite of these, sperm count and sperm morphology remained normal in the EMF exposed group. Meistrich11 reported that evaluation of sperm counts seems to be a good indicator of spermatogenic damages. From these conflicting data, the decrease in the number of spermatocyte in the Group E2 might be considered to be an accidental finding. Moreover, the same change was not seen in the Group E1. However, we could observe another interesting finding that the Leydig cell count in Group E2 was significantly higher than that in Group E1 and the control.
According to Holm et al.,12 Leydig cell hyperplasia is associated with impaired spermatogenesis. Leydig cell clusters were found more frequently in biopsies associated with impaired spermatogenesis and the presence of micro-nodules seems to be a histological marker of testicular failure in men. Thus, the hyperplasia of Leydig cells would be expected to have important influences on the restoration of normal spermatogenesis.13 This interpretation was strengthened by the fact that there was concomitant increase in serum testosterone levels in the Group E2. It was previously reported that testosterone alone could induce spermatogenesis and produce normally fertile spermatozoa in the absence of circulating gonadotropins.14 Moreover, the androgenic threshold to maintain spermatogenesis in mice is much lower than the threshold required for inducing spermatogenesis.15 These data demonstrate that chronic exposure throughout the sexually immature period to microwave frequencies and power density level used in our daily lives substantially affects the reproductive ability of males, but clinically relevant effects of EMF radiation on the reproductive system is unlikely to occur.
de Seze et al.16 showed that radiocellular telephones do not disturb the secretion of anterior pituitary hormones in humans, although the authors did not correlate them with testosterone levels. In our study, serum LH and FSH did not show a significant change between EMF irradiated and sham exposed groups. Interestingly, Park et al.17 reported the immediate increase of LH in rabbits following irradiation with 2.45 GHz microwave for 30 minutes. However, this sudden rise of LH seems to have influenced by the stress originated from the high density EMF of sub-lethal dose. Our study focused on to eliminate subsidiary changes derived from stress or thermal effects by adopting low density EMF and could not find any significant difference in LH level.
In conclusion, the reduction of spermatocytes in rats exposed to EMF suggests that prolonged exposure to low-level EMF may have negative effects on spermatogenesis that presumably deteriorates fertilization. However, Leydig cell hyperplasia followed by an elevated level of serum testosterone may play an important role in restoring normal spermatogenesis. However, it is necessary to conduct further studies under different experimental conditions, to clarify the effect of EMF exposure.
After long-term exposure to electromagnetic field radiation on rat's testis, we found decrease of spermatocyte and Leydig cells proliferation. And it was proved by increase of serum testosterone.
These changes show that electromagnetic field radiation gives negative effects on spermatogenesis. And we assumed that proliferation of Leydig cells is important role to compensatory activation of spermatogenesis to maintain normal state. For certification of this estimation, more long term studies about radiation dose and exposed duration and reversibility in electromagnetic field are needed.
Figures and Tables
 | Fig. 1(A) Electromagnetic field shield room- the exterior view. (B) Electromagnetic field exposure facility inside the shield room. |
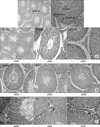 | Fig. 2(A) Histology of the ipsilateral testis in the control rats. H&E, reduced from ×100, ×200, ×400. (B) Histology of the testes in Control, E1 and E2 groups. Note that the number of spermatocytes is decreased in group E2 compared to the control group. H&E, reduced from ×200. (C) Histology of the testes in the control, E1 and E2 groups. Note that the number of Leydig cells (Pink arrow) is increased in group E2 compared to the control group. H&E, reduced from ×400. |
 | Fig. 3Serum hormonal level in each group after electromagnetic field (EMF) radiation. Values are reported as mean±SDs. (A) Testosterone was significantly increased in Group E2 compared to the control group. *Significant difference compared to the control group (p<0.01). (B) Luteinizing hormone (LH) did not show any significant difference between the groups. (C) Follicle stimulating hormone (FSH) did not show any significant difference between the groups. |
Notes
References
1. Brent RL. Reproductive and teratologic effects of low-frequency electromagnetic fields: a review of in vivo and in vitro studies using animal models. Teratology. 1999. 59:261–286.
2. Kowalczuk CI, Saunders RD, Stapleton HR. Sperm count and sperm abnormality in male mice after exposure to 2.45 GHz microwave radiation. Mutat Res. 1983. 122:155–161.
3. Johnsen SG. Testicular biopsy score count - a method for registration of spermatogenesis in human testis: normal values and results in 335 hypogonadal males. Hormones. 1970. 1:2–25.
4. Saunders RD, Kowalczuk CI. Effects of 2.45 GHz microwave radiation and heat on mouse spermatogenic epithelium. Int J Radiat Biol Relat Stud Phys Chem Med. 1981. 40:623–632.
5. Dewhirst MW, Lora-Michiels M, Viglianti BL, Dewey WC, Repacholi M. Carcinogenic effects of hyperthermia. Int J Hyperthermia. 2003. 19:236–251.
6. Blackwell RP. Standards for microwave radiation. Nature. 1979. 282:360.
7. Nagler HM, White RD. The effect of testicular torsion on the contralateral testis. J Urol. 1982. 128:1343–1348.
8. Hecht K, Balzer HU. Biological effects of electromagnetic fields on humans in the frequency range of 0 to 3 GHz. 1997. Berlin: I.S.F.;1–4.
9. Collins P, Lacy D. Studies on the structure and function of the mammalian testis. IV. Steroid metabolism in vitro by isolated interstitium and seminiferous tubules of rat testis after heat sterilization. Proc R Soc Lond B Biol Sci. 1974. 186:37–51.
10. Varma MM, Traboulay EA Jr. Biological effects of microwave radiation on the testes of Swiss mice. Experientia. 1975. 31:301–302.
11. Meistrich ML. Evaluation of reproductive toxicity by testicular sperm head counts. J Am Coll Toxicol. 1989. 8:551–566.
12. Holm M, Rajpert-De Meyts E, Andersson AM, Skakkebaek NE. Leydig cell micronodules are a common finding in testicular biopsies from men with impaired spermatogenesis and are associated with decreased testosterone/LH ratio. J Pathol. 2003. 199:378–386.
13. Hand JW, Walker H, Hornsey S, Field SB. Effects of hyperthermia on the mouse testis and its response to X-rays, as assayed by weight loss. Int J Radiat Biol Relat Stud Phys Chem Med. 1979. 35:521–528.
14. Singh J, O'Neill C, Handelsman DJ. Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology. 1995. 136:5311–5321.
15. Handelsman DJ, Spaliviero JA, Simpson JM, Allan CM, Singh J. Spermatogenesis without gonadotropins: maintenance has a lower testosterone threshold than initiation. Endocrinology. 1999. 140:3938–3946.
16. de Seze R, Fabbro-Peray P, Miro L. GSM radiocellular telephones do not disturb the secretion of antepituitary hormones in humans. Bioelectromagnetics. 1998. 19:271–278.
17. Park CY, Nam DS, Kim SH, Shin HJ, Lee JH, Bae JH, et al. Hormonal (cortisol, growth hormone, luteinizing hormone, thyroid stimulating hormone) changes of rabbits exposed to microwaves. J Korean Neurosurg Soc. 1996. 25:920–928.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download