Abstract
Purpose
In this study, we tested whether injections of muscle-derived stem cells and alginate (Alg)/polycaprolactone (PCL) after denervation of the pudendal nerve could increase the leak point pressure (LPP) and closing pressure (CP) over the long term in a rat model of urinary incontinence.
Materials and Methods
Muscle derived stem cells (MDSC) were isolated from the gastrocnemius muscle of normal female rats, and these cells were purified for creating a myogenic population by the preplate technique. In the denervated (D) group, the pudendal nerve was transected bilaterally via a dorsal incision in order to denervate the external urethral sphincter. The denervated external urethral sphincter was injected with Alg/PCL (AP group), or MDSC/Alg/PCL (M group) into the proximal urethra after pudendal nerve transection. At 1 and 3 months, the LPP and CP measurements were visually identified by using the vertical tilt/intravesical pressure clamp model of stress urinary incontinence. The rats were then sacrificed and their urethras were harvested for histology.
Results
Both the LPP and CP were significantly lower in the denervated group at each time compared with the normal (N group), AP and M groups, and both the LPP and CP in the N, AP and M groups were significantly higher than those in the D group at both 1 and 3 months. The persistence of MDSC over the period of the study was verified by histology. Thus, pudendal nerve denervation led to a progressive decline in the LPP and CP that was evident at 1 month and this persisted to 3 months, and injection of MDSC/Alg/PCL into the denervated rats led to a long term increase in the LPP and CP.
Conclusions
The N, AP and M groups all had significantly higher LPPs than the D group, and MDSC/Alg/PCL injection into the denervated external urethral sphincter in female rats increased the LPP and CP in both the short and long term. We also observed a long term bulking effect of MDSC/Alg/PCL injection in the stress incontinence animal model.
Figures and Tables
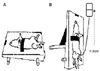 | Fig. 1Vertical tilt/intravesical pressure clamp model of stress urinary incontinence. (A) Acute spinal cord transection at the T9-T10 level was performed with the rat in a supine position and a transvesical catheter with a fire-flared tip (PE-90) was inserted into the dome of the bladder for bladder filling and pressure recording. (B) The intravesical pressure was varied in 1-3cm H2O steps from zero upward until we visually identified the leak point. The height was then adjusted downward until the leaking stopped. |
 | Fig. 2Leak point pressure (LPP) and closing pressure (CP) at 1 & 3 months. The LPP and CP in the D group were significantly lower at 1 & 3 month than the LPP and CP in the N, AP, and M groups. The LPP and CP in the AP and M groups were significantly higher than that in the denervation group at 1 & 3 months. N: normal, D: denervation, AP: alginate (Alg)/polycaprolactone (PCL), M: muscle derived stem cell. *: p<0.01 compared to N group, †: p<0.01 compared to D group. |
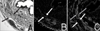 | Fig. 3Histology of the normal urethral sphincter of female rats. (A) Hematoxylin/eosin staining at 3 months. In the normal female rat urethral sphincter, a layer of striated muscle fibers (black arrow) encircles the smooth muscle layers (gray arrow). (B) MyHC immunostaining of the 3 months specimen. In the normal female rat urethral sphincter, the skeletal muscle (white arrow) shows positive DAPI and MyHC staining. (C) a-SMA immunostaining of the 3 months specimen. Smooth muscle (gray arrow) shows positive a-SMA staining, and the skeletal muscle (white arrow) shows blanks (A, B, C ×100). |
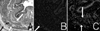 | Fig. 4Histology of the denervated urethral sphincter of female rat. (A) Hematoxylin/eosin staining at 3 months. The denervated proximal urethral sphincter also showed atrophic and thin circular skeletal fibers (black arrow). The smooth muscle is well preserved (white arrow). (B) MyHC immunostaining of the 3 months specimen. The denervated proximal urethral sphincter skeletal muscle (white arrow) shows negative MyHC staining. (C) a-SMA immunostaining of the 3 months specimen. Smooth muscle (white arrow) shows positive DAPI and a-SMA staining (A, B, C ×100). |
 | Fig. 5Histology of the Alg/PCL injected urethral sphincter of female rat. (A) Hematoxylin/eosin staining at 3 months. The Alg/PCL injection area (Alg: white arrow, PCL: black arrow) is well preserved. (B) MyHC immunostaining of the 3 months specimen. Ingrowing muscular bundles (white arrow) are observed on the periphery of the polymer. (C) a-SMA immunostaining of the 3 months specimen. The organized smooth muscle layers (white arrow) shows positive DAPI and a-SMA staining, but ingrowing muscular bundles on the periphery of the polymer (gray arrow) are observed as negative a-SMA staining (A, B, C ×100). |
 | Fig. 6Histology of the MDSC/Alg/PCL injected urethral sphincter of female rat. (A) Hematoxylin/eosin staining at 3 months. The MDSC/Alg/PCL injection area (Alg: gray arrow, PCL: black arrow) is well preserved. (B) Expression of PKH26 at the 3 months specimen. The MDSC injection area was PKH26 positive. (C) a-SMA immunostaining of the 3 months specimen. Muscular bundles (white arrow) are observed to be positive for a-SMA staining on the periphery of the polymer. (D) Merging of DAPI and, PKH26 (A, B, C, D ×100). |
References
1. Appell RA, Dmochowski RR, Herschorn S. Urethral injections for female stress incontinence. BJU Int. 2006. 98:Suppl 1. 27–30.
2. Chapple CR, Brubaker L, Haab F, van Kerrebroeck P, Robinson D. Patient-perceived outcomes in the treatment of stress urinary incontinence: focus on urethral injection therapy. Int Urogynecol J Pelvic Floor Dysfunct. 2007. 18:199–205.
3. Lee JY, Cannon TW, Pruchnic R, Fraser MO, Huard J, Chancellor MB. The effects of periurethral muscle-derived stem cell injection on leak point pressure in a rat model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2003. 14:31–37.
4. Cho SH, Oh SH, Lee JH. Fabrication and characterization of porous alginate/polyvinyl alcohol hybrid scaffolds for 3D cell culture. J Biomater Sci Polym Ed. 2005. 16:933–947.
5. Oh SH, Kang SG, Kim ES, Cho SH, Lee JH. Fabrication and characterization of hydrophilic poly (lactic-co-glycolic acid)/poly (vinyl alcohol) blend cell scaffolds by melt-molding particulate-leaching method. Biomaterials. 2003. 24:4011–4021.
6. Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002. 157:851–864.
7. Lee JY, Paik SY, Yuk SH, Lee JH, Ghil SH. The isolation and characterization of muscle derived stem cells from gastrocnemius muscle of rats using the modified preplate method. Korean J Urol. 2004. 45:1279–1284.
8. Phelan M, Fraser MO, Yokoyama T. The vertical tilt table and intravesical pressure clamp: new animal models for the study of continence mechanisms and detrusor hyperreflexia. J Urol. 2001. 165:Suppl. 416A.
9. Conway DA, Kamo I, Yoshimura N, Chancellor MB, Cannon TW. Comparison of leak point pressure methods in an animal model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2005. 16:359–363.
10. Cannon TW, Wojcik EM, Ferguson CL, Saraga S, Thomas C, Damaser MS. Effects of vaginal distension on urethral anatomy and function. BJU Int. 2002. 90:403–407.
11. Chermansky CJ, Cannon TW, Torimoto K, Fraser MO, Yoshimura N, de Groat WC, et al. A model of intrinsic sphincteric deficiency in the rat: electrocauterization. Neurourol Urodyn. 2004. 23:166–171.
12. Cannon TW, Lee JY, Somogyi G, Pruchnic R, Smith CP, Huard J, et al. Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology. 2003. 62:958–963.
13. Lee JY, Paik SY, Yuk SH, Lee JH, Ghil SH, Lee SS. Long term effects of muscle-derived stem cells on leak point pressure and closing pressure in rats with transected pudendal nerves. Mol Cells. 2004. 18:309–313.
14. Oh SH, Lee JY, Ghil SH, Lee SS, Yuk SH, Lee JH. PCL microparticle-dispersed PLGA solution as a potential injectable urethral bulking agent. Biomaterials. 2006. 27:1936–1944.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download