Abstract
Purpose
We wanted to compare the efficacy and toxicity of chemotherapy with methotrexate, vinblastine, adriamycin, cisplatin (M-VAC) versus gemcitabine and cisplatin (GC) for patients with advanced or metastatic urothelial carcinoma.
Materials and Methods
Forty-nine patients diagnosed with advanced urothelial cell carcinoma and who were started on chemotherapy were divided into two groups. All of them had a 0-1 Eastern Cooperative Oncology Group performance status. 19 patients received M-VAC chemotherapy and 30 patients received the GC regimen. Among them, the subjects who completed more than 3 cycles of their recommended formula (13/19 for M-VAC, 28/30 for GC) were included in this study. They were evaluated for their overall response rate, the 5-year survival rate, toxicities and the drop-out rate.
Results
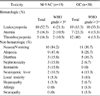
The overall response rate and median survival period of the M-VAC and G-C groups were 38% (5/13 cases) and 46% (13/28 cases), and 16.7 months and 43.9 months, respectively. The 5-year survival rates in the two groups were 10% in the M-VAC group and 46% in the G-C treated group (p=0.013). The main hematologic complication was leukopenia and this occurred in 10/19 patients and more than grade 3 leukopenia was noted in 4/10 patients in the M-VAC group and in 19/30 patients and more than grade 3 was noted in 10/19 patients in the GC group. The common non-hematologic side effects between the two groups were nausea/vomiting (84.2% vs 47.7%), alopecia (47.4% vs 26.7%), diarrhea (15.8% vs 16.7%), and nephrotoxicity (15.8% vs 6.7%), respectively. The drop-out rates were 31.6% in the M-VAC group and 6.7% with the GC group.
Figures and Tables
 | Fig. 15-year survival graph according to methotrexate, vinblastine, adriamycin, cisplatin (M-VAC) and gemcitabine and cisplatin (GC) chemotherapy. |
 | Fig. 2Overall survival graph according to the cancer site. (A) upper urinary tract, (B) lower urinary tract. GC: gemcitabine and cisplatin, M-VAC: methotrexate, vinblastine, adriamycin and cisplatin. |
References
1. Sternberg CN, Yagoda A, Scher HI, Watson RC, Herr HW, Morse MJ, et al. M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for advanced transitional cell carcinoma of the urothelium. J Urol. 1988. 139:461–469.
2. Tannock I, Gospodarowicz M, Connolly J, Jewett M. M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) chemotherapy for transitional cell carcinoma: the Princess Margaret Hospital experience. J Urol. 1989. 142:289–292.
3. Pectasides D, Pectasides E, Economopoulos T. Systemic therapy in metastatic or recurrent endometrial cancer. Cancer Treat Rev. 2007. 33:177–190.
4. Jung JH, Lee SJ, Kim HW, Cho SY, Kim SW, Lee CB, et al. Efficacy and toxicity of gemcitabine plus cisplatin chemotherapy in advanced urothelial cancer. Korean J Urol. 2003. 44:739–744.
5. Yoon DJ, Chang SG. Efficacy and toxicity of gemcitabine based chemotherapy for advanced urothelial cancer. Korean J Urol. 2002. 43:7–13.
6. Jang CS, Cho JS, Lee SK, Lee YG, Yang DY, Kim SY, et al. Hematologic toxicity of gemcitabine and cisplatin combination therapy in advanced urothelial cancer. Korean J Urol. 2003. 44:672–676.
7. Khaled HM, Hamza MR, Mansour O, Gaafar R, Zaghloul MS. A phase II study of gemcitabine plus cisplatin chemotherapy in advanced bilharzial bladder carcinoma. Eur J Cancer. 2000. 36:Suppl 2. 34–37.
8. Yagoda A. Progress in treatment of advanced urothelial tract tumors. J Clin Oncol. 1985. 3:1448–1450.
9. Igawa M, Ohkuchi T, Ueki T, Ueda M, Okada K, Usui T. Usefulness and limitations of methotrexate, vinblastine, doxorubicin and cisplatin for the treatment of advanced urothelial cancer. J Urol. 1990. 144:662–665.
10. Dimopoulos MA, Finn L, Logothetis CJ. Pattern of failure and survival of patients with metastatic urothelial tumors relapsing after cis-platinum-based chemotherapy. J Urol. 1994. 151:598–600.
11. Roth BJ. Chemotherapy for advanced bladder cancer. Semin Oncol. 1996. 23:633–644.
12. Saxman SB, Propert KJ, Einhorn LH, Crawford ED, Tannock I, Raghavan D, et al. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. 1997. 15:2564–2569.
13. Lee CK, Choi S, Kim JC, Rhew HY. A retrospective study comparing M-VAC and CISCA chemotherapy in advanced urothelial transitional cell carcinoma. Korean J Urol. 1994. 35:1339–1346.
14. Dodd PM, Bajorin DF. New chemotherapy regimens for metastatic bladder cancer. Curr Opin Urol. 1998. 8:413–418.
15. Sternberg CN. Gemcitabine in bladder cancer. Semin Oncol. 2000. 27(1):Suppl 2. 31–39.
16. Kaufman D, Raghavan D, Carducci M, Levine EG, Murphy B, Aisner J, et al. Phase II trial of gemcitabine plus cisplatin in patients with metastatic urothelial cancer. J Clin Oncol. 2000. 18:1921–1927.
17. von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000. 18:3068–3077.
18. Stadler WM, Hayden A, von der Maase H, Roychowdhury D, Dogliotti L, Seymour L, et al. Long-term survival in phase II trials of gemcitabine plus cisplatin for advanced transitional cell cancer. Urol Oncol. 2002. 7:153–157.
19. Bajorin DF, Dodd PM, Mazumdar M, Fazzari M, McCaffrey JA, Scher HI, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999. 17:3173–3181.
20. Loehrer PJ, Einhorn LH, Elson PJ, Crawford ED, Kuebler P, Tannock I, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. 1992. 10:1066–1073.
21. Geller NL, Sternberg CN, Penenberg D, Scher H, Yagoda A. Prognostic factors for survival of patients with advanced urothelial tumors treated with methotrexate, vinblastine, doxorubicin, and cisplatin chemotherapy. Cancer. 1991. 67:1525–1531.
22. Als AB, Sengelov L, von der Maase H. Long-term survival after gemcitabine and cisplatin in patients with locally advanced transitional cell carcinoma of the bladder: focus on supplementary treatment strategies. Eur Urol. 2007. 52:478–486.
23. Moore MJ, Winquist EW, Murray N, Tannock IF, Huan E, Bennett K, et al. Gemcitabine plus cisplatin, an active regimen in advanced urothelial cancer: a phase II trial of the National Cancer Institute of Canada Clinical Trial Group. J Clin Oncol. 1999. 17:2876–2881.
24. Stadler WM, Kuzel T, Roth B, Raghavan D, Dorr FA. Phase II study of single-agent gemcitabine in previously untreated patients with metastatic urothelial cancer. J Clin Oncol. 1997. 15:3394–3398.
25. Roberts JT, von der Maase H, Sengelov L, Conte PF, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine/cisplatin and methotrexate/vinblastine/doxorubicin/cisplatin in patients with locally advanced and metastatic bladder cancer. Ann Oncol. 2006. 17:Suppl 5. v118–v122.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download