Abstract
Purpose
This study was designed to examine the effect of stress on the pathophysiology of bladder stability via the Rho-kinase and nitric oxide synthase activity, which are required for muscle contraction and relaxation within the bladder.
Materials and Methods
Animal testing was conducted in two separate sessions. In the first experiment, 36 female Sprague-Dawley rats weighing about 230-270g each were employed. 18 rats were placed in the control group and 18 rats were placed in the test group. The second testing was conducted using metabolic cages. Six rats were placed in the control group and six rats were placed in the test group under a stressful environment.
Results
The results showed that the frequency of urination was significantly increased with time in the test group (p<0.05). However, the volume of voided urine decreased, thereby suggesting stress was a cause of overactive bladder. Analysis of bladder tissue for nitric oxide synthase (NOS) and RhoA-binding kinase (ROKα), important components of contraction and relaxation of bladder muscle, revealed that the levels of iNOS and ROKα were significantly increased with continued application of stress. This suggests that stress affects the levels of NOS and ROKα in an overactive bladder to influence contraction and relaxation of the bladder muscle.
Figures and Tables
 | Fig. 1Effect of stress on the urinary bladder function test parameters. (A) Voiding frequency: This figure shows the total frequency of voiding within 3 hours, (B) Voiding volume: These graphs presented volume (µl)/void in the stress and control groups with prolonged application of the stress (*: p<0.05, †: p<0.01). |
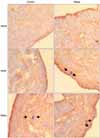 | Fig. 2Pattern of the DAB positive area in bladder tisssue after immunohistochemical staining for nNOS, iNOS and ROKα at 20 days. Magnification 200x (C: urothelium, D: submucosa, E: muscularis mucosa) DAB: diamino benzidine, nNOS: neuronal nitric oxide synthase, iNOS: inducible nitric oxide synthase, ROKα: rhoA-binding kinase. |
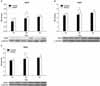 | Fig. 3Western blotting assay of bladder tissue for determining the protein expression level (*: p<0.05). (A) Western blotting assay for nNOS expression. (B) Western blotting assay for iNOS expression. (C) Western blotting assay for ROKα expression (nNOS: neuronal nitric oxide synthase, iNOS: inducible nitric oxide synthase, ROKα: rhoA-binding kinase). |
 | Fig. 4RT-PCR analysis of bladder tissue for determining the expression level of the mRNA of ROKα transcript (*: p<0.05) RT-PCR: reverse trnascript-polymerase chain reaction, ROKα: rhoA-binding kinase. |
Table 1
Stimuli used for induction of stress in rats by limiting the cage size and decreasing the ambient temperature and accessibility to food and water

References
1. Kyrou I, Chrousos GP, Tsigos C. Stress, visceral obesity, and metabolic complications. Ann N Y Acad Sci. 2006. 1083:77–110.
2. Yang SK. Psycho-urology: possible links between stress, anxiety, depression and bladder function. J Korean Continence Soc. 2006. 10:1–8.
3. Bigal ME, Lipton RB. Modifiable risk factors for migraine progression. Headache. 2006. 46:1334–1343.
4. Crofford LJ. Violence, stress, and somatic syndromes. Trauma Violence Abuse. 2007. 8:299–313.
5. Sand PK, Appell R. Disruptive effects of overactive bladder and urge urinary incontinence in younger women. Am J Med. 2006. 119:3 Suppl 1. 16–23.
6. Clemens JQ, Brown SO, Kozloff L, Calhoun EA. Predictors of symptom severity in patients with chronic prostatitis and interstitial cystitis. J Urol. 2006. 175:963–966.
7. Glover L, Gannon K, McLoughlin J, Emberton M. Men's experiences of having lower urinary tract symptoms: factors relating to bother. BJU Int. 2004. 94:563–567.
8. Cukier JM, Cortina-Borja M, Brading AF. A case-control study to examine any association between idiopathic detrusor instability and gastrointestinal tract disorder, and between irritable bowel syndrome and urinary tract disorder. Br J Urol. 1997. 79:865–878.
9. Morrison LM, Eadie AS, McAlister A, Glen ES, Taylor J, Rowan D. Personality testing in 226 patient with urinary incontinence. Br J Urol. 1986. 58:387–389.
10. Walters MD, Taylor S, Schoenfeld LS. Psychosexual study of women with detrusor instability. Obstet Gynecol. 1990. 75:22–26.
11. Engstrom G, Henningsohn L, Steineck G, Leppert J. Self-assessed health, sadness and happiness in relation to the total burden of symptoms from the lower urinary tract. BJU Int. 2003. 95:810–815.
12. McGhan WF. Cost effectiveness and quality of life considerations in the treatment of patients with overactive bladder. Am J Manag Care. 2001. 7:2 Suppl. S62–S75.
13. Gatenbeck L, Johansson B, Strömberg L, Svensson M. Genital blood flow in male rats subjected to stress stimuli. Urol Res. 1987. 15:297–301.
14. Yoon H, Chung WS, Park YY, Cho IH. Effects of stress on female rat sexual function. Int J Impot Res. 2005. 17:33–38.
15. Cabral CA, Sampaio FJ, Cardoso LE. Analysis of the modifications in the composition of bladder glycosaminoglycan and collagen as a consequence of changes in sex hormones associated with puberty or oophorectomy in female rats. J Urol. 2003. 170:2512–2516.
16. Shapiro E, Becich MJ, Perlman E, Lepor H. Bladder wall abnormalities in myelodysplastic bladders: a computer assisted morphometric analysis. J Urol. 1991. 145:1024–1029.
17. Kato K, Wein AJ, Radzinski C, Longhurst PA, McGuire EJ, Miller LF, et al. Short term functional effects of bladder outlet obstruction in the cat. J Urol. 1990. 143:1020–1025.
18. Chitaley K, Wingard CJ, Clinton Webb R, Branam H, Stopper VS, Lewis RW, et al. Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide independent pathway. Nat Med. 2001. 7:119–122.
19. Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science. 1996. 273:245–248.
20. Wibberley A, Chen Z, Hu E, Hieble JP, Westfall TD. Expression and functional role of Rho-kinase in rat urinary bladder smooth muscle. Br J Pharmacol. 2003. 138:757–766.
21. Rajasekaran M, Mehta N, Baquir A, Kuntz S. Rho-kinase inhibition suppresses potassium chloride-induced bladder hyperactivity in a rat model. Urology. 2007. 69:791–794.
22. Rajasekaran M, Wilkes N, Kuntz S, E Albo M. Rho-kinase inhibition suppresses bladder hyperactivity in spontaneously hypertensive rats. Neurourol Urodyn. 2005. 24:295–300.
23. Damaser MS, Whitbeck C, Barreto M, Horan P, Benno H, O'connor LJ, et al. Comparative physiology and biochemistry of rat and rabbit urinary bladder. BJU Int. 2000. 85:519–525.
24. Wang P, Luthin GR, Ruggieri MR. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J Pharmacol Exp Ther. 1995. 273:959–966.
25. Yildiz A, Hayirli A, Okumus Z, Kaynar O, Kisa F. Physiological profile of juvenile rats: effects of cage size and cage density. Lab Anim. 2007. 36:28–38.
26. Demir A, Onol FF, Ercan F, Tarcan T. Effect of cold-induced stress on rat bladder tissue contractility and histomorphology. Neurourol Urodyn. 2007. 26:296–301.
27. Cetinel S, Ercan F, Cikler E, Contuk G, Sener G. Protective effect of melatonin on water avoidance stress induced degeneration of the bladder. J Urol. 2005. 173:267–270.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download